The periodic table | Chemistry for Grade 10 PDF Download
Mendeleev's periodic table
Early attempts to classify elements
- The atomic weight of an element is equivalent to what we now call its relative atomic mass.
- Early periodic tables were incomplete, since many elements were unknown. Also, some elements were placed in groups with elements that were not similar to them.
Dmitri Mendeleev
- Dmitri Mendeleev was a Russian chemist. He wrote chemistry books and was looking for ways to organise the known elements. He published his first periodic table of the elements in 1869. In it, he arranged the elements in order of increasing atomic weights. He also took into account the properties of the elements and their compounds. This meant that his table:
- had gaps in it
- showed elements with similar chemical properties lined up in groups
- However, from their atomic weights, some pairs of elements next to each other were in the wrong order.
Predictions using gaps
- Mendeleev left gaps in his table for elements not known at the time. By looking at the properties of the elements next to a gap, he could also predict the properties of these undiscovered elements. For example, Mendeleev predicted the existence of 'eka-silicon', which would fit into a gap below silicon. Another scientist later discovered the missing element, germanium. Its properties were found to be similar to the predicted ones and confirmed Mendeleev's periodic table.
Pair reversals
- Iodine has a lower atomic weight than tellurium. So iodine should be placed before tellurium in Mendeleev's periodic table. However, iodine has similar chemical properties to chlorine and bromine. To make iodine line up with chlorine and bromine in his table, Mendeleev swapped the positions of iodine and tellurium.

The modern periodic table
In the modern periodic table:
- elements are arranged in rows, called periods, in order of increasing atomic number
- elements with similar properties are placed in vertical columns, called groups
The table is called the periodic table because elements with similar properties occur at regular intervals.
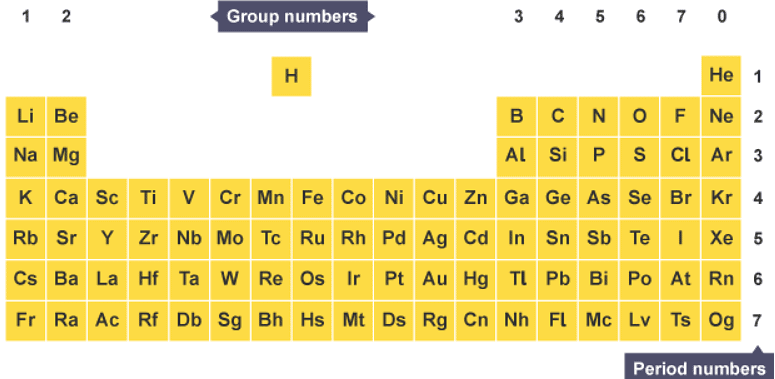
The modern periodic table with some elements left out for simplicity
Resolving pair reversals
- Mendeleev did not know about isotopes, but their existence explains pair reversals. The positions of iodine and tellurium were reversed in Mendeleev's table because:
- iodine has one naturally occurring isotope, iodine-127
- the most abundant tellurium isotopes are tellurium-128 and tellurium-130
- The high relative abundance of these tellurium isotopes gives tellurium the greater relative atomic mass. The atomic number of tellurium is 52 and the atomic number of iodine is 53, so these elements are in the correct order in the modern periodic table.
Electronic structure
- An electronic structure is the way in which electrons are arranged in an atom.
Electrons in shells
- Electrons in atoms occupy energy levels, also called electron shells, outside the nucleus. Different shells can hold different maximum numbers of electrons. The electrons in an atom occupy the lowest available energy level first. This is the shell nearest the nucleus. When this shell is full the electrons begin to occupy the next energy level.
- Below is a table showing the maximum number of electrons an element can have for each of its energy level shells. The information shown is for elements with atomic numbers 1 to 20:

Predicting an electronic structure
- The electronic structure of an atom can be predicted from its atomic number. For example, the atomic number of sodium is 11. Sodium atoms have 11 protons and so 11 electrons:
- two electrons occupy the first shell
- eight electrons occupy the second shell
- one electron occupies the third shell
- This electronic structure can be written as 2,8,1 (each comma, or dot, separates one shell from the next). This electronic structure can also be shown as a diagram. In these diagrams:
- each shell is shown as a circle
- each electron is shown as a dot or a cross

The electronic structure of sodium as a diagram
Electronic structures and the periodic table
- The electronic structure of an element is linked to its position on the periodic table.

The electronic structure of sodium (2,8,1) shows that sodium, Na:
- is in period 3
- is in group 1
- has an atomic number of (2 + 8 + 1) = 11
Properties of metals and non-metals
Differences in chemical properties
Most elements are metals. In their chemical reactions, metal atoms lose electrons to form positive ions. For example:
- when magnesium burns in air, each atom loses two electrons to form a Mg2+ ion
- when sodium reacts with chlorine, each sodium atom loses one electron to form a Na+ ion

A sodium atom, Na, forms a Na+ ion by losing an electron.
- Elements that do not form positive ions in their chemical reaction are non-metals.
- For more information on ions, see the study guide on ions.
- The chemical properties of the compounds of metal and non-metal elements are also different:
- most metal oxides are basic
- most non-metal oxides are acidic
Differences in physical properties
- Metal and non-metal elements have different physical properties:
- most metals have high melting and boiling points
- most non-metals have low melting and boiling points
- The table shows some other differences in physical properties of metals and non-metals, when solid.

Metals and non-metals in the periodic table
In the periodic table:
- metal elements are on the left of a stepped line starting at B-Al-Si
- non-metal elements are on the right of the stepped line
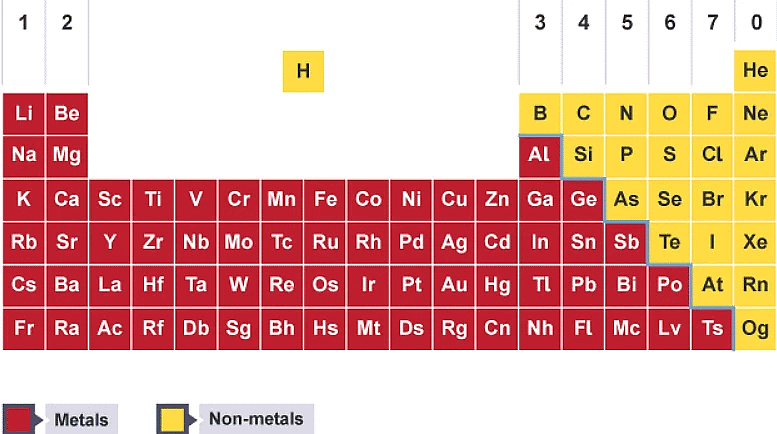
Metals are on the left of the periodic table, and non-metals are on the right.
Atomic structure and the periodic table
- Elements in group 1 and group 2 are metals. Atoms of group 1 elements have one electron in their outer shell, and atoms of group 2 elements have two electrons in their outer shell.
- Elements in groups 6, 7 and 0 are non-metals. Atoms of group 7 elements have seven electrons in their outer shell, and atoms of group 0 elements, except helium, have eight electrons in their outer shell.
- The reactions of elements are related to the number of electrons in their outer shells:
- Atoms of metal elements give away electrons in their reactions to form positive ions. The ions formed have a full outer electron shell, so are very stable.
- Atoms of non-metal elements gain electrons in some of their reactions to form negative ions. The ions formed have a full outer electron shell, so are very stable.
|
78 videos|87 docs|11 tests
|

















