Grade 11 Exam > Grade 11 Notes > Chemistry for Grade 11 (IGCSE) > Water: Chemical Tests
Water: Chemical Tests | Chemistry for Grade 11 (IGCSE) PDF Download
Chemical tests for water
Using cobalt(II) chloride
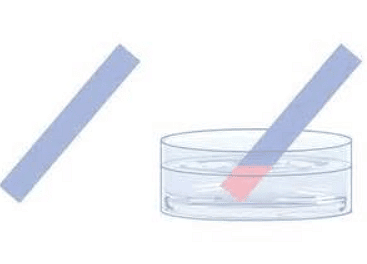
- Cobalt(II) chloride changes color from blue to pink when water is added. This test is commonly conducted with cobalt chloride paper. The chemical equation for this process involves anhydrous cobalt(II) chloride reacting with water to form hydrated cobalt(II) chloride.
- The equation is:
anhydrous cobalt(II) chloride + water ⇌ hydrated cobalt(II) chloride
CoCl2 (s) + 6H2O (l) ⇌ CoCl2.6H2O (s) - Test for water using cobalt chloride paper which turns pink in the presence of water:

Using Copper(II) Sulfate
- Anhydrous copper(II) sulfate changes from white to blue in the presence of water.
- The chemical equation for this reaction is:
anhydrous copper(II) sulfate + water ⇋ hydrated copper(II) sulfate
CuSO4 (s) + 5H2O (l) ⇋ CuSO4.5H2O (s) - Test for water using anhydrous copper(II) sulfate which turns blue in the presence of water:

Purity of Water
Testing for purity
- Pure substances melt and boil at specific and sharp temperatures
- Water has a boiling point of 100 °C and a melting point of 0 °C
- Mixtures have a range of melting and boiling points as they consist of different substances that melt or boil at different temperatures
- Melting and boiling points data can therefore be used to determine the purity of water
- Impurities tend to increase the boiling point of water, so impure water will boil at temperatures above 100 °C
- Impurities tend to decrease the melting point of water, so impure water will melt at temperatures below 0 °C
Distilled Water
- Distilled water is created by heating water to form a vapor, then condensing it back into a liquid state.
- It is characterized by a very low presence of impurities, making it highly pure.
- In practical chemistry, distilled water is preferred due to its high purity levels.
- Tap water, on the other hand, contains more impurities which could potentially disrupt chemical reactions, hence it is not commonly used in labs.
Question for Water: Chemical TestsTry yourself:Which chemical test can be used to detect the presence of water?
View Solution
The document Water: Chemical Tests | Chemistry for Grade 11 (IGCSE) is a part of the Grade 11 Course Chemistry for Grade 11 (IGCSE).
All you need of Grade 11 at this link: Grade 11
|
103 docs|53 tests
|
FAQs on Water: Chemical Tests - Chemistry for Grade 11 (IGCSE)
| 1. How can you test the purity of water using chemical tests? |  |
Ans. Chemical tests such as pH test, chlorine test, turbidity test, conductivity test, and total dissolved solids test can be used to determine the purity of water.
| 2. What does a pH test reveal about the water's purity? |  |
Ans. A pH test can indicate the acidity or alkalinity of water, with pure water having a pH of 7. Deviations from this value may suggest contamination or impurities.
| 3. Why is a chlorine test important in assessing water purity? |  |
Ans. A chlorine test helps determine the presence of chlorine in water, which is commonly used for disinfection. High levels of chlorine may indicate over-chlorination or contamination.
| 4. How does turbidity testing help in assessing water purity? |  |
Ans. Turbidity testing measures the cloudiness or haziness of water caused by suspended particles. High turbidity levels can indicate the presence of contaminants or pollutants.
| 5. What information can be obtained from a conductivity test for water purity? |  |
Ans. Conductivity testing measures the ability of water to conduct electricity, which is influenced by the presence of dissolved ions. High conductivity levels may suggest contamination or mineral content in water.

|
Explore Courses for Grade 11 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
Related Searches