Class 8 Science Chapter 11 Important Question Answers - Chemical Effects of Electric Current
Q1: Define good conductors and poor conductors or insulators.
Ans: The materials that conduct electricity through them are called good conductors whereas those that do not conduct electricity are called poor conductors or insulators. For example, copper, brass, aluminium, iron, etc., are conductors whereas rubber, plastic, wood, air, etc., are insulators.
Q2: Give an example of the chemical effect of the electric current.
Ans: The electric current through a conducting solution causes chemical reactions as a result, bubbles of gas are formed, deposits of metal are seen on electrodes or changes in colour of solution, may occur. These are some of the chemical effects of electric current.
Q3: Which effect of electric current is utilized for detecting the flow of current through a solution
(a) When a torch bulb is used?
(b) When a magnetic compass is used?
Ans: (a) Heating effect
(b) Magnetic effect.
Q4: Distilled water does not conduct electricity. What substances can be added to distilled water in small amounts to make it a good conductor of electricity?
Ans: Pure water or distilled water does not conduct electricity. In distilled water there are no impurities, there areno ions, there are only neutral (no charge) water molecules and these neutral molecules do not have charge, but after adding salt/ acid/ base, it starts conducting due presence of ions.
Q5: In case of a fire, before the fireman uses the water hoses to throw water to douse the fire, they shut off the electricity supply for the area. Explain why this is done?
Ans: Water usually contains impurities which makes it a good conductor of electricity. If the electrical supply is not shut off, then electricity may pass through water and harm the firemen. This is the reason in case of a fire before the firemen use the water hoses; they shut off the main electrical supply for the area.
Q6:What is electroplating? What are its uses?
Ans: The process of depositing a layer of any desired metal on another material by means of electricity is called electroplating.
Electroplating is a very useful process. This is used to make objects appear shiny and resistant to scratches. It prevents corrosion. For example, iron used in bridges and automobiles is coated with zinc to protect them from corrosion and formation of rust.
Q7: What effects does electric current produce when flowing through a conducting solution?
Ans:When an electric current flows through the conducting solution, it causes a chemical reaction (or chemical change).
These chemical reactions may produce the following effects:
- Bubbles of gas or gases may be formed on the electrodes.
- Deposits of metals may form on electrodes.
- Change in colour of solution may occur.
Q8:What are the advantages and disadvantages of electroplating?
Ans: Advantages:
- It protects the metals from being corroded.
- It prevents the rusting of metals.
- It makes cheap and dull metals shiny and attractive.
- It can make more reactive metals like iron less reactive.
- Chromium coating on metals give lustre to objects.
Disadvantages
- Pollutants from electroplating industries are very harmful. Some chemicals are very lethal for both human and animals.
- It is an expensive process.
Q9: Which effect of electric current is utilized when a thin layer of chromium metal is deposited on an iron tap? What is this process known as?
Ans: Chemical effect of current is utilized when a thin layer of chromium metal is deposited on an iron tap. The process is known as electroplating. Chromium has a shiny look. It does not get corroded and it resists scratches. Chromium is however expensive and it may not be economical to make the whole object out of it. So the object is made from a cheaper metal and only a coating of chromium is done over it.
Q10: Which metal is electroplated on iron for making ‘cans’ used for storing food & Why?
Ans:Tin is electroplated on iron for making ‘cans’ used for storing food. It has a shiny appearance and does not corrode. Also tin is non-poisonous as it is less reactive than iron. Due to tin plating over the surface of iron, the food does not come in contact with iron and is protected from getting spoilt.
Q11: What is an LED? Why is it preferred to other type of bulbs?
Ans: The electric device which is used in the tester instead of bulb is an LED. Its full form is Light Emitting Diode. It is preferred to other bulbs as it can glow even when weak or less current flows through it.
Q12: What happens when electric current is passed through the copper sulphate solution?
Ans: When electric current is passed through the copper sulphate solution, copper sulphate dissociates into copper and sulphate. The free copper gets drawn to the electrode connected to the negative terminal of the battery and gets deposited on it.
Q13: On what factors the chemical effect produced by an electric current depends?
Ans:The chemical effect produced by an electric current depends on the nature of conducting solution (through which it is passed) and on the nature of electrodes used for passing the electric current.
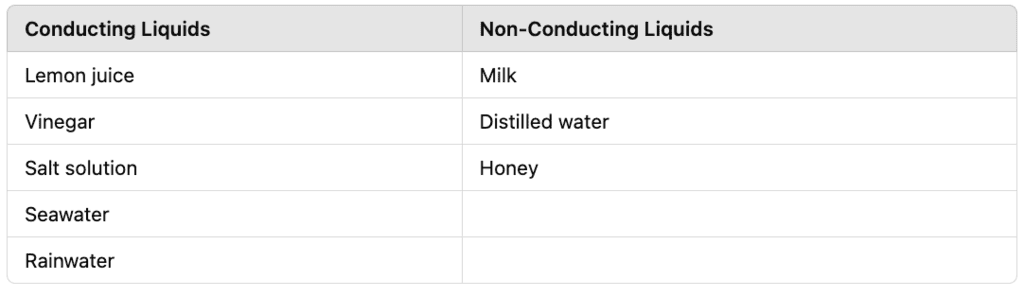
Q14: Which of the following liquids conduct electricity and which do not conduct electricity?
Lemon juice, Milk, Vinegar, Salt solution, Distilled water, Honey, Seawater, Rainwater.
Ans:
 Q15: What is the magnetic effect of electricity? Explain.
Q15: What is the magnetic effect of electricity? Explain.
Ans: When electric current is passed through a coil or wire, it behaves like a magnet. This is known as magnetic effect of current. It depends on amount of current passing through the coil or wire.
|
136 videos|530 docs|57 tests
|
FAQs on Class 8 Science Chapter 11 Important Question Answers - Chemical Effects of Electric Current
| 1. What is the chemical effect of electric current? |  |
| 2. How does an electric current cause chemical changes? |  |
| 3. What are some examples of chemical effects of electric current? |  |
| 4. How is the chemical effect of electric current used in practical applications? |  |
| 5. Can the chemical effect of electric current be harmful? |  |

















