Class 9 Science Chapter 3 Case Based Questions - Atoms and Molecules
(I) Read the following passage and answer the questions based on the passage and related studied concepts.
Chemical reactions follow laws of chemical combination such as law of conservation of mass, law of constant (definite) proportion. Atom is smallest particle of an element that retains all its chemical properties and takes part in chemical reaction. Molecule is made of elements or compounds, capable of independent existence. It shows all properties of substance. A chemical formula of compound show its elements and number of atoms of each element. Cluster of atoms act as polyatomic ions having fixed charge on them and value helps to decide chemical formula. Atoms of C–12 are assigned relative atomic mass–12 and relative mass of all other atoms are determined with the help of C–12. The Avogadro’s number 6.022 × 1023 is defined as the number of atoms in exactly 12 g of carbon 12. Mole is amount of substance that contains same number of atom as 12 g of C–12. Mass of 1 mole of substance is called its molar mass.
Q1: Calculate the percentage of oxygen in CO2. [Atomic mass of C = 12 u, O = 16 u]
Ans: % of oxygen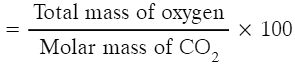


Q2: How many mole of atoms are present in 10 g of calcium atom? [Ca = 44u]
Ans: Number of moles
Number of atoms = Number of mole × 6.022 × 1023 = 0.25 × 6.022 × 1023 = 1.505 × 1023 atoms.
Q3: 0.25 mole of an element ‘X’ is 9.75 g. What is X ?
Ans: 0.25 mole of X = 9.75 g
1 mole of X = 9.75 ÷ 0.25 = 39.0 g mol–1
The element is Potassium.
Q4: What is empirical formula of a compound with composition 80% copper and 20% sulphur. [At. mass of Cu = 64 u, S = 32 u]
Ans: 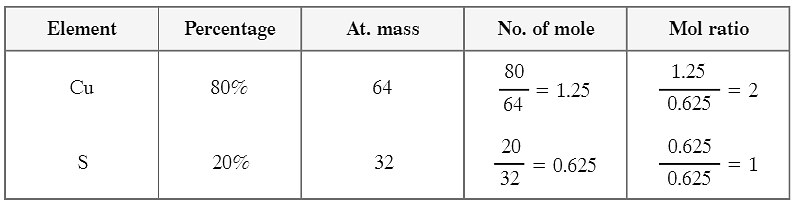
The empirical formula of the compound is Cu2S.
(II) Table shows common ions with fixed charges Answer the questions based on this table and related studied concepts.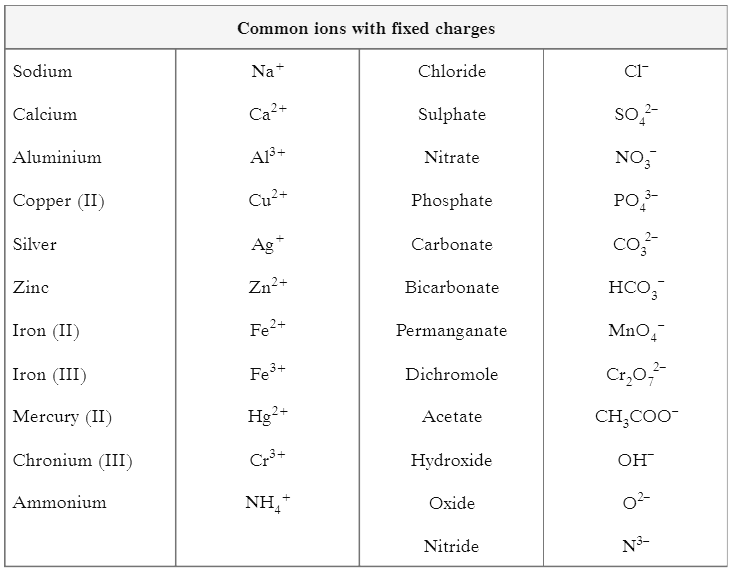
Q1: What is formula of magnesium nitride?
Ans:

Q2: Calculate the molar mass of Iron (II) Sulphate. [Fe = 56 u, S = 32 u, O = 16 u]
Ans: 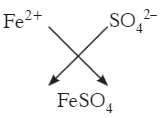
Molar mass of FeSO4 = 56 + 32 + (16 × 4) = 152 g mol–1
Q3: Write name of (NH4)2SO4
Ans: Ammonium sulphate.
Q4: Give one example of polyatomic anion.
Ans: CO32-(Carbonate).
|
88 videos|369 docs|67 tests
|


















