Electronic Effects: Inductive, Hyperconjugation & Resonance | Organic Chemistry PDF Download

Electron rich species (8 e– species having lone pair/negative charge) | Electron deficient species (6 e– species having vacent p or d orbital) |
1. Anions: Cl–, Br–, CN–, –OH, –NH2, RO–, R– | 1. Species having vacant p orbitals: BeCl2, BF3, BCl3,BBr3, Carbocations, Carbene, Nitrene, Benzyne.
are not electrophiles. |
 | 2. Species having vacant d orbitals: PCl3, PCl5, SiF4,SnCl4, ZnCl2, FeCl3. Cations: Zn+2, Cu+2, Pt+2, Fe+2, Ni+2, Cr+3 |
3.Alkenes: Alkenes having Electron Donating groups such as —OH, —OR, —NH2, —NR2, PhH, Alkynes. | 3. Alkenes: Alkenes having Electron Withdrawing groups such as —NO2, —CN. |
4.Organometallic: RLi, RMgX, R2CuLi, R2Zn [R has negative charge on it in all of them] | 4. Ketenes:  |
5. Ambident: Having two donor sites  | 5. Carbonyls: Carbon atom of carbonyl group has δ+ charge, hence, attack of nucleophiles can take place.
|
Role: Based upon the mode and site of attack Electron rich species can act as following- (a) Nucleophile (b) Base (c) Ligand (d) Reducing agent (e) HOMO (highest occupied molecular orbital) | Role: Based upon the mode and site of attack Electron deficient species can act as following- (a) Electrophile (b) Acid (c) Transition metal (d) Oxidizing agent (e) LUMO (lowest unoccupied molecular orbital) |
Ambiphiles: Those reagents which can act as both electrophile and nucleophile.  | |
Strength and Reactivity: Strength and reactivity of all electron rich species and electron deficient ones depend upon various Electronic effects (electronic displacement in covalent bonds).
- Electronic effects are divided into two categories:
A. Primary Effects
B. Secondary Effects

A. Primary Effects
1. Electronegativity: Higher is the electronegativity difference, lower is the pKa value and hence higher acidity.

2. Size of Atom: As size of central atom increases, pKa value decreases because negative charge so developed due to loss of proton, spreads over large sized atom.

B. Secondary Effects

A. Polarisation Effects:
1. Inductive Effect: It is permanent, distance dependent effect which only operates through σ-bond. C—C bond in ethane is non polar while this (C—C) bond has got significant amount of polarity in Chloroethane.

The influences of this induction of polarity in a relatively nonpolar σ-bonds by the adjacent polar σ-bond is known as Inductive effect.

This effect weakens steadily with increasing distance from the substituent and actually dies down after three carbon atoms. There are two types of Inductive effects.
(a) Electron withdrawing Inductive Effect (–I Effect): Substituents which are more electronegative with respect to H, then it will have –I effect.

–I Groups
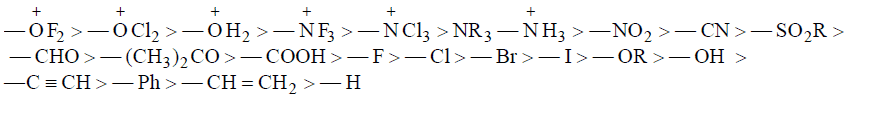
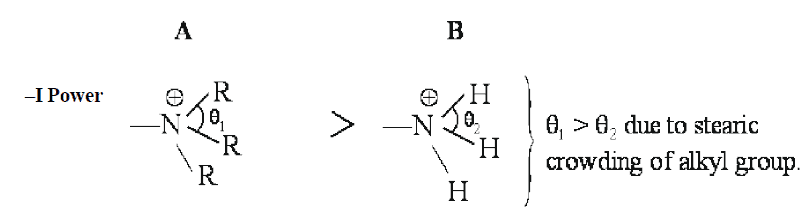
Since θ1 > θ2 ,that is why quaternary nitrogen (A) is more electronegative than ammonium nitrogen (B). Therefore, quaternary nitrogen (A) shows more –I effect than ammonium ion (B).
Here, primary factor (electronegativity of the nitrogen atom) playing more dominating role than +I effect of alkyl group (R).

(Considering steric factor because the size of nitrogen is small)
(b) Electron Donating Inductive Effect (+I Effect): If the substituent attached is less electronegative than H, it will release electron away from itself and hence it will have +I effect.

Applications of Inductive Effect
(i) Effect of bond lengths: Since the inductive effect leads to ionic character in the bond, the increase in –I effect usually decrease the bond length.
Increasing –I effect

(ii) Dipole Moment: Since, inductive effect leads to a diplolar character in the molecule, it developes some dipole moment in the molecule, which increases with the increase in the inductive effect.
CH3—I < CH3—Br < CH3—Cl
Increasing dipole moment.
(iii) Reactivity of alkyl halides: Alkyl halides are more reactive than the corresponding alkanes due to presence of C—X bond which is polar due to I effect, furthermore reactivity increases with increase of branching.

Increasing reactivity due to increase C—X bond polarity.
(iv) Strength of carboxylic acids: Strength of an acid depends upon the ease with which an acid ionizes to give proton. A molecule of carboxylic acid can be represented as a resonance hybrid of the following structures.
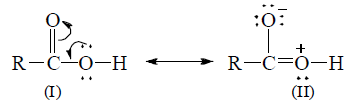
In the II structure, the oxygen atom of the hydroxyl group has a positive charge due to which it has a tendency to attract electron pair (inductive effect) of the O—H bond towards itself, which results in the removal of hydrogen atom as proton and hence carboxylic acids behave as acids. Once, the carboxylate anion is formed, it is stablized more easily by resonance than undissociated acid.

Thus, the acidity of carboxylic acid is due to inductive effect and resonance stabilization of the carboxylate anion. Thus any group or atom, which is highly electronegative help in removing the hydrogen atom as proton and the group or atom which is less electronegative than C marks the removal of proton difficult. Hence (–I) effect group increases acidic strength and (+1) effect groups decreases the acidic strength of carboxylic acid.
(V) Basic Strength of Amines: The basic character of amines is due to presence of unshared electron pair on nitrogen atom which accepts proton; the readiness with which the lone pair of electrons available for protonation determines strength of amines.
Due to +I effect of alkyl group, the nitrogen atom becomes rich in electrons with the result the lone pair of electron on nitrogen atom in amiens is more easily available than in ammonia and hence generally, alphatic amines are stronger bases than ammonia.
On the other (–I) groups or electron groups attached to nitrogen atom makes it difficult for protonation.
Note: Relative basic strength of amines is not in total accordance witht the inductive effect, other factors like sterric effect and stabilisation of cation by hydration also play importance role to determine the basic strength of amines.
2. HYPERCONJUGATION
Bond Resonance or σ - π electron Delocalization)
Spreading out charge by the overlap of an empty p orbital with an adjacent filled orbital is called hyperconjugation. Hyperconjugation involves electron delocalization (via partial orbital overlap) from a filled bonding orbital to an adjacent unfilled orbital.
Occurrance: Allene, Alkynes, Free radicals (saturated type) carbonium ions (saturated type)
Condition: Presence of α—H with respect to cation, double bond, triple bond, carbon containing positive charge (in carbonium ion) or unpaired electron (in free radicals).
(1) The involvement of σ BMO with π* ABMO (σ Work as HOMO & π* work as LUMO).
(2) It may involve overlapping between σ BMO (filled) with empty p orbital.
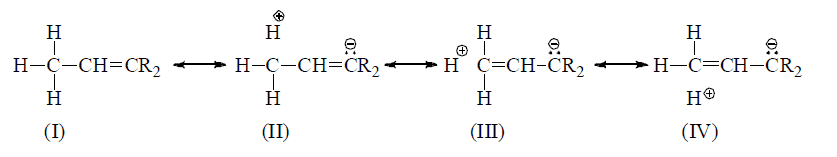
Note: Number of hyperconjugative structures = number of α—H hence, in above example structures, II, III,IV are hyperconjugative structures (H-structures)
When an unsaturated system or unshared orbitals (e.s., carbocation or carbon free radical) has α—H the compound of this catagory will have various hyperconjugative structures like contributing various hyperconjugative structures like contributing structures and α—π electron delocalization is possible.

Note: Permanet effect, operates through α—π electron delocalization normally hyperconjugative effect is weaker than Resonance effect.
Application:
(i) Bond Length: Like resonance, hyperconjugation also affects bond length because during the process the single bond in compound acquires some double bond character and vice-versa. E.g. C—C bond length in propene is 1.488 Å as compared to 1.334 Å in ethylene.

(ii) Dipole Moment: Since hyperconjugation causes the development of charges, it also affects the dipole moment of the molecule.
(ii) Stability of carbocations
The order of stability of carbonicarbocation is as follows
Tertiary > Secondary > Primary
Above order of stability can be explained by hyperconjugation. In general greater the number of hydrogen atoms attached to α- carbon atoms, the more hyperconjugative forms can be written and thus greater will be the stability of carbonium ions.
Illustration: Among (I), (II) & (III) which carbocation is more stable.

Ans. Stability order: (III ) > (II ) > (I)

(v) Orientation influence of methyl group: the o, p-directing of the methyl group in methyl benzene is ttributed partly to inductive and partly of hyperconjugation effect.
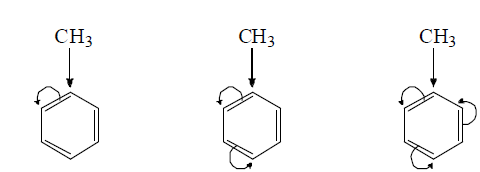
(Orientation incluence of the methyl group due to +I effect)
The role of hyperconjugation in o, p-directing incluence of methyl group is evidenced by the fact that nitrogen of p-isopropyl toluence and p-tert-butyl toluene form the product in which NO2 group is introduced in the ortho position with respect to methyl group and not to isopropyl or t-butyl group althrough the latter groups are more electron donating than

Methyl group i.e., The substitution takes place contrary to inductive effect. Actually this constitutes an example where hyperconjugation overpowers inductive effect.
(vi) Stability of alkenes: Stability of alkenes can be explained by hyperconjugative effect.

The most common intermediates formed are radicals, carbocations and carbanions etc.
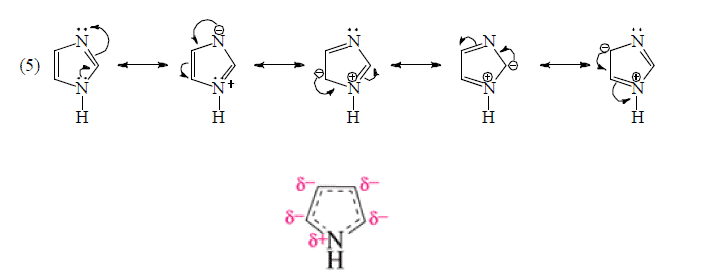
3. RESONANCE ( π -Electron delocalization)
When two or more Lewis structures (valence-bond structures) are possible, differing only in the placement of electrons, the molecule will usually show characteristics of both structures.
The structures are called Resonating structures or contributing structures because they are not different comopunds, just different ways of drawing the same compound. The actual molecule is said to be a resonance hybride of its contributing structures. NO+ (Nitrosonium) might be represented by either of the following resonance forms.

Resonance hybrid:
The difference structures of a compound devised by different methods of pairing electrons in a fixed atomic skeleton are called resonance or canonical structures. The actual structure of the compound is then a combination of these structures and hence the compound is called a resonance hybrid. A hybrid is more stable than any one of the contributing structures.
A. Molecules Exhibiting Resonance
(a) Conjugated multiple bonds
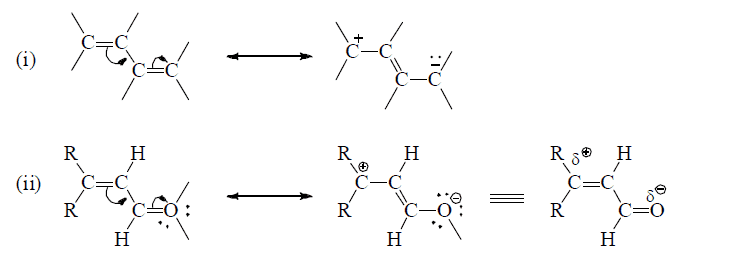
(b) Multiple bond in conjugation with an unshared p-orpital on an adjacent atom.

Effective sidewise overlap between π- electrons and unshared orbital having electron pair.


Rules for writing Resonating Structures.
(a) All resonating structures must be valid Lewis structure.

(b) Positions of atomic nuclei and s-electrons remain constant. Resonance is all about π-electron delocalization therefore position of π-electrons and non-bonding electrons may change in various resonating states.

Invalid resonance states for benzene because distance between two carbon atoms are changing in these structure.
(c) All the resonating structure must have same number of unpaired electrons (i.e., Δn=0)
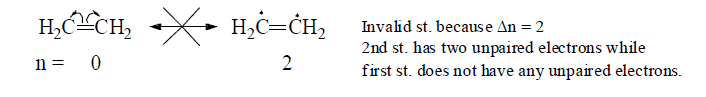
(d) For the effective overlap of p-orbitals, part of the molecule taking part in resonance must be plannar.

B. Relative contribution of resonating structures
(a) Octet Completion
(a) Neutral structure more stable than charged structure. [May be fail only if aromatic character]
(b) Structures must have complete octet of all the atoms and more no. of bonds.
(c) Fries rule: More stable if more no. of benzene rings
(d) AMRHI concept is very useful find out relative stability of resonating structures:
A. Aromatic E. Mesomeric R. Resonance
H. Hyper-conjugation I. Inductive effect
(b) Greater the number of covalent bonds higher the contributiion towards resonance hybrid.
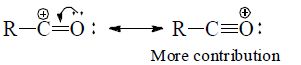

(c). –ve charge is stable at atom with high %S character
(d) Resonating structure which places –ve charge on more electronegative atom is more contributing while resonating structure which places +ve change on less electronegative atom are more contributing.
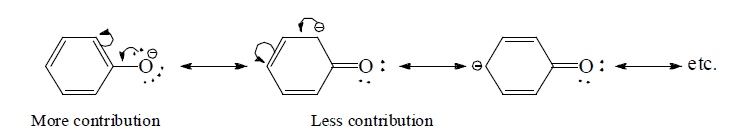
(e) Structures with positive charge on multiply bonded electronegative element are unimportant since the electronegative element will not adapt to such distribution of electrons. Hence the canonical form,  of carbonyl group is insignificant.
of carbonyl group is insignificant.
(f) Charge separated resonating sts. are less contributing as compared to non charge/separated resonating structure.

(g) Charge-separation structures are less important than those in which the charge is delocalized. This is due to electrostatic attraction between unlike charges

Hence, acetic acid favors ionization.
- Dissimilar canonical structures vary widely in the energy contents, those of higher energy contribute too little to the hybrid. In such case the lowest-energy structure resembles the hybrid in energy, i.e., the RS(resonating structure) is less e.g.,
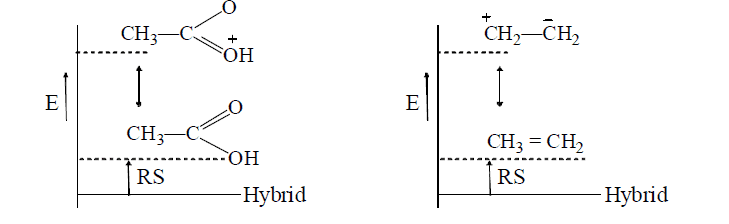
Hence, acetic acid and ethylene are well represented by their conventional structures.
- In contrast, similar canonical structures have nearly equal energies and contribute more to the hybrid, so that the RS is more, e.g.
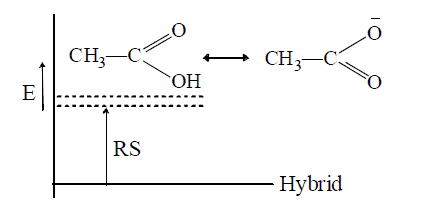
Thus, acetate ion is more stable than acetic acid which therefore ionizes to behave as acid. Similarly, phenol is acidic since phenoxide ion is more stable than phenol (see +M effect)
(h) Contribution is insignificant when identical charges on two adjacent atoms are present.

Application of above rules for more stable resonating structure:

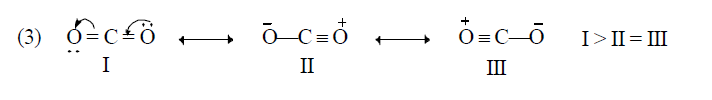
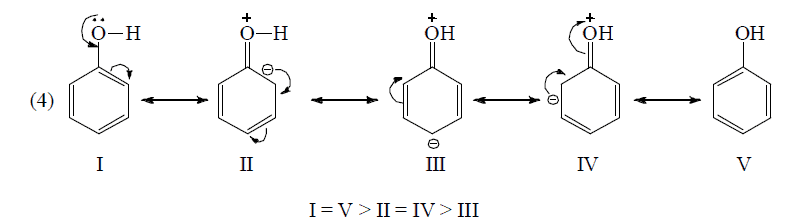
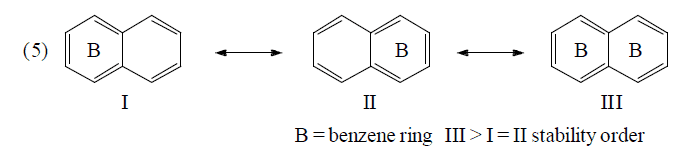
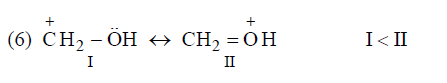
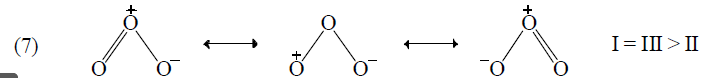
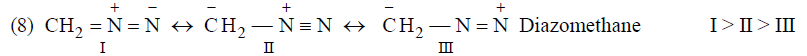
Resonance Energy
The difference of energy between the resonance bybrid (actual molecule) and the most stable contributing structure is known as resonance energy.
The greater the number of significant structures that can be written and the more nearly equal they are, the greater the resonance energy.



D. Types of resonance on the basis of functional groups
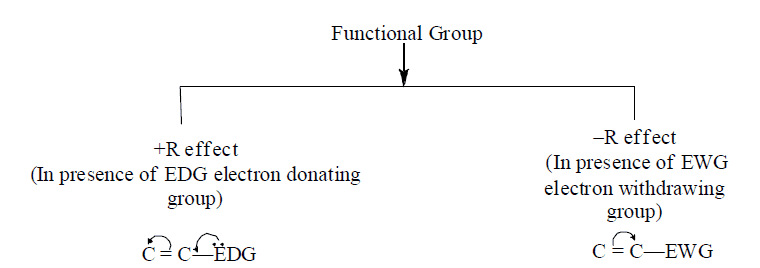
1. +R and –R effect take place equally at ortho and para position
2. I-effect is distance dependent and order is ortho > meta > para.



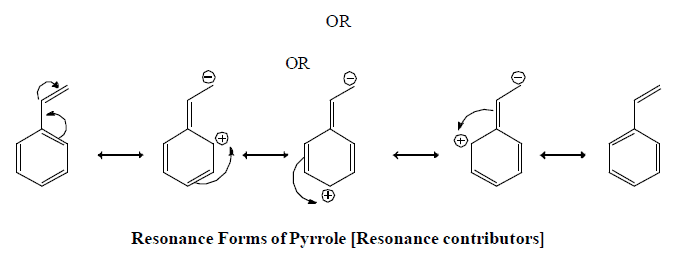
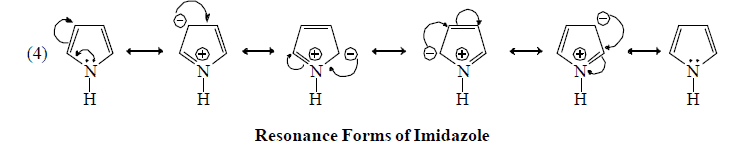





Resonating form of Benzyl Carbocation

|
35 videos|92 docs|46 tests
|
FAQs on Electronic Effects: Inductive, Hyperconjugation & Resonance - Organic Chemistry
| 1. What is the inductive effect? |  |
| 2. What is hyperconjugation? |  |
| 3. What is resonance effect? |  |
| 4. How do electronic effects influence the acidity of a compound? |  |
| 5. How does the inductive effect differ from the resonance effect? |  |

|
Explore Courses for Chemistry exam
|

|





















