Entropy Calculations for Ideal Gases | Thermodynamics - Mechanical Engineering PDF Download
Entropy Calculations for Ideal Gases
For a closed system, the first law provides:
dU = dQ + dW
If the process of change occurs under reversible conditions then the above equation becomes:
d ( H − PV) = TdS − PdV
dH − PdV − VdP= TdS − PdV ............(4.18)
However for an ideal gas: dH = CigpdT .....(4.19)
Thus combining eqns. 4.18 and 4.19:

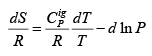 .....(4.20)
.....(4.20)
Integrating between an initial (T0, P0) and any final (T, P):
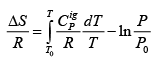
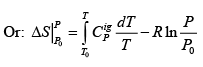 .....(4.21)
.....(4.21)
The last equation provides a direct expression for computing entropy change between two states for an ideal gas.
For the special case of a reversible adiabatic dQ = 0; hence dS= 0. Thus:
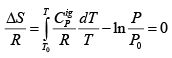
For constant CPig , it follows that: 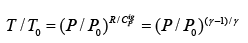 .....(4.22)
.....(4.22)
One may note that eqn. 4.22 provides the same relation as obtained by the Fist Law analysis as in eqn. 3.18.
|
29 videos|65 docs|36 tests
|
FAQs on Entropy Calculations for Ideal Gases - Thermodynamics - Mechanical Engineering
| 1. What is entropy and how is it calculated for ideal gases? |  |
| 2. How does the entropy of an ideal gas change with temperature? |  |
| 3. Can the entropy of an ideal gas be negative? |  |
| 4. How does the entropy of an ideal gas change with pressure? |  |
| 5. What is the significance of entropy in thermodynamics and engineering? |  |

|
Explore Courses for Mechanical Engineering exam
|

|

















