Classification of Elements & Periodicity in Properties Class 11 Notes Chemistry Chapter 3
Why Do We Need To Classify Elements?
Elements serve as the fundamental building blocks of all forms of matter. In 1800, only 31 elements had been identified, but by 1865, this number had more than doubled to 63. At present, 118 elements are known. With such a large number of elements, it is very difficult to study individually the chemistry of all these elements and their number of compounds. We need to classify elements for these reasons:
1. Organization:
- Classification helps organize the vast array of elements into a meaningful system.
- The periodic table arranges elements based on atomic number and properties.
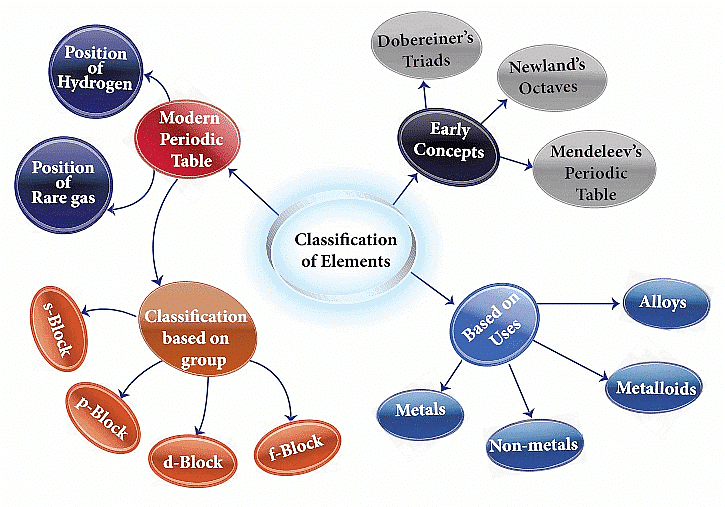
 Classification of Elements
Classification of Elements
2. Predicting properties:
- Classification allows us to predict an element's properties based on its position in the periodic table.
- Elements within the same group often exhibit similar behavior.
3. Discovering new elements: Classification helps identify gaps in the periodic table, guiding efforts to discover new elements.
4. Communication and referencing: Classifying elements provides a standardized framework for scientists to communicate and reference elements.
5. Education: Element classification is a fundamental concept in chemistry education, providing a foundation for further learning.
Overall, classifying elements enables organization, prediction, discovery, effective communication, and education in the field of chemistry.
Genesis of Periodic Classification
Various attempts were made to classify elements, such as 'Dobereiner's Triads,' 'Newland's Octaves,' Lothar Meyer Classification, and Mendeleev's Periodic Table. These efforts paved the way for the formulation of the Modern Periodic Law and the development of the present form of the periodic table. Let's delve into each of these classifications to gain a deeper understanding.
Note: The atomic weights of the elements were used in all previous attempts to classify them.
1. Dobereiner’s Triads
German chemist Johann Wolfgang Dobereiner attempted to classify elements with similar properties into groups of three elements each. These groups were called ‘triads’.
- Dobereiner suggested that in these triads, the atomic mass of the element in the middle would be more or less equal to the mean of the atomic masses of the other two elements in the triad.
- An example of such a triad would be one containing lithium, sodium, and potassium. The atomic mass of lithium is 6.94 and that of potassium is 39.10. The element in the middle of this triad, sodium, has an atomic mass of 22.99 which is more or less equal to the mean of the atomic masses of lithium and potassium (which is 23.02).
The Limitations of Dobereiner’s Triads are:
- Newly discovered elements did not fit into the triads.
- Only a total of 5 Dobereiner’s triads were identified.
- Even several known elements did not fit into any of the triads.

2. Newland’s Octaves
English scientist John Newlands arranged the 56 known elements in increasing order of atomic mass in the year 1866.
 Newland's Law of Octaves
Newland's Law of Octaves
- Newland’s Law of Octaves states that when the elements are arranged in increasing order of atomic mass, the periodicity in properties of two elements which have an interval of seven elements in between them would be similar.
- He observed a trend wherein every eighth element exhibited properties similar to the first.
Limitations of Newland’s octaves are:
- Several elements fit into the same slots in Newland’s periodic classification. For example, cobalt and nickel were placed in the same slot.
- Elements with dissimilar properties were grouped together. For example, the halogens were grouped with some metals such as cobalt, nickel and platinum.
- Newland’s law of octaves held true only for elements up to calcium. Elements with greater atomic masses could not be accommodated into octaves.
- The discovery of noble gases added to the limitations. Therefore, this method of classifying elements did not leave any room for the discovery of new elements.
3. Lothar Meyer’s Curve
According to Lothar Meyer, atoms with similar properties occupy similar places in the atomic volume, as measured by the atomic mass curve. He stated that “The atomic masses of elements are periodic functions of their physical properties.”
 Lothar Meyer
Lothar Meyer
- Meyer considered the volume taken up by fixed weights of the various elements. Each weight contained the same number of atoms of its particular element (Avogadro's number). This meant that the ratio of the volumes of the various elements was equal to the ratio of the volumes of single atoms of the various elements.
- Thus Lothar Meyer could determine the atomic volumes of elements. If the atomic volumes of the elements were plotted against the atomic weight, a series of peaks were produced.
- The peaks had alkali metals: sodium, potassium, rubidium, and cesium. Each fall and rise to a peak, corresponded to a period like the waves.
- In each period a number of physical properties other than atomic volume also fell and rose, such as valence and melting point. The second and third period in Meyer's table included seven elements each, and duplicated Newlands' law of octaves.
Limitations of Lothar Meyer’s Classification
- Meyer's periodic table was insufficient in comparison to Mendeleev's periodic table, which was published the same year and made remarkable predictions about the discovery of certain elements.
- Meyer's classification was supported by a study of various physical properties related to atomic weights with no empirical or logical basis or classification, and such values are difficult to remember. Mendeleev's periodic classification, on the other hand, was founded on Periodic Law.
4. Mendeleev’s Periodic Table and Law
Mendeleev organised atoms in a table's horizontal rows and vertical columns in order of increasing atomic weights, so that elements with comparable properties were grouped together in the same vertical column. Mendleev's Periodic Table
Mendleev's Periodic Table
- The Periodic Law (also referred to as Mendeleev’s Law), states that the chemical properties of elements are a periodic function of their atomic weights.
- The elements with similar properties come into the same group.
- Mendeleev also left gaps in his periodic table for undiscovered elements like aluminium, silicon and Boron in his periodic table and named them Eka-Aluminium, Eka-silicon and Eka-Boron.
- Mendeleev not only predicted the existence of Eka-Aluminium, Eka-silicon, and Eka-Boron but also described the general physical properties of these elements.
- These elements were discovered later and named Gallium, Germanium, and Scandium.
- Mendeleev's periodic table could predict the properties of several elements based on their position in the periodic table.
- Mendeleev's periodic table could accommodate noble gases when they were discovered.
Limitations of Mendeleev's periodic table:
- The position of isotopes could not be explained.
- The wrong order of atomic masses of some elements could not be explained.
- The position of Hydrogen could not be assigned in a periodic table.
Modern Periodic Table
When Mendeleev formulated his Periodic Table, scientists did not know the internal structure of atoms. However, significant advancements in sub-atomic particle theories occurred at the beginning of the 20th century.
- In 1913, the English physicist Henry Moseley observed regularities in the characteristic X-ray spectra of elements. By plotting the frequency of emitted X-rays (ν) against the atomic number (Z), he demonstrated that the atomic number is a more fundamental characteristic of an element than its atomic mass, unlike the plot of ν versus atomic mass.
Modern Periodic Law
- Periodic Law statement: The modern periodic law states that the properties of elements are periodic functions of their atomic numbers.
- Atomic number: The atomic number, which represents the number of protons in an atom's nucleus, is the fundamental factor in determining an element's position in the periodic table.
 Modern Periodic Table
Modern Periodic Table
Present Form of the Periodic Table
- Structure: The present form of the periodic table is a tabular arrangement of elements based on their atomic numbers, electronic configurations, and chemical properties.
- Periods: The table is divided into periods (rows) that represent the successive energy levels or shells of electrons surrounding the nucleus.
- Groups: The table is also divided into groups (columns) based on similar properties and valence electron configurations. Elements within the same group often exhibit similar chemical behavior.
- Classification: Elements are classified into several categories, including metals, nonmetals, and metalloids, based on their properties.
- Periodic trends: The periodic table allows for the observation of various periodic trends, such as atomic radius, ionization energy, electron affinity, and electronegativity, which change systematically across periods and groups.
- Transition metals: The transition metals are placed in a block in the middle of the periodic table, and they exhibit similar properties due to their partially filled d orbitals.
- Lanthanides and actinides: The lanthanides and actinides, also known as the inner transition metals, are placed below the main body of the periodic table to conserve space. They have unique electron configurations and exhibit similar properties within each series.
The present form of the periodic table provides a systematic and organized representation of elements, enabling scientists to study their properties, predict behavior, and make meaningful connections between elements based on their atomic structure and periodic trends.
Nomenclature of Elements With Atomic Numbers > 100
1. The name of the element is directly derived from its atomic number by using the following numerical roots:
- 0 = nil (n)
- 1 = un (u)
- 2 = bi (b)
- 3 = tri (t)
- 4 = quad (q)
- 5 = pent (p)
- 6 = hex (h)
- 7 = sept (s)
- 8 = oct (o)
- 9 = enn (e)
2. The numerical roots are put together according to the digits, which make up the atomic number and end with “ium” to spell out the name.
3. The element symbol consists of the initial letters of the numerical roots, which make up the name.
4. In the name of the element, each root is pronounced separately such that the root ‘un’ should get pronounced with a long ‘u’.
For example, let us find the name of an element with atomic number 120:
Code for 1 is un
Code for 2 is bi
Code for 0 is nil
Hence the name of the element with atomic number 120 is un + bi + nil + ium (ending code), that is; unbinilium.
However, it is necessary to note that if the last digit code like bi ends with the letter ‘i’ then just add ‘um’. So, the ending, in that case, will be ‘um’ instead of ‘ium’.
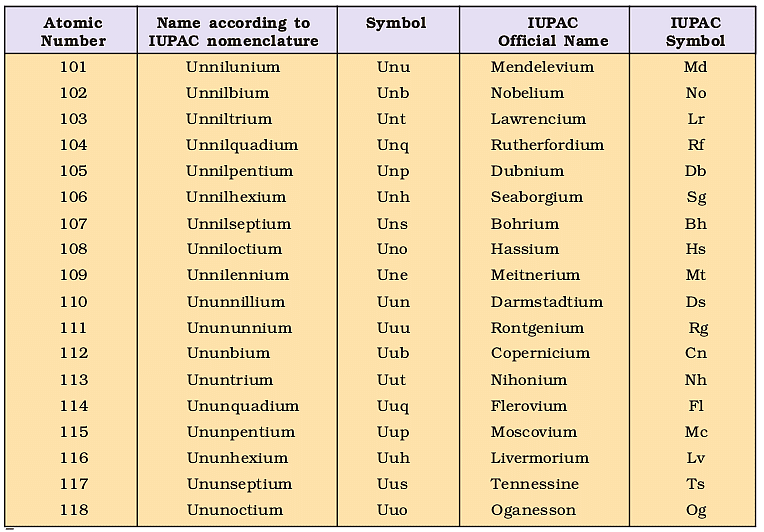
IUPAC Nomenclature Of Elements With Atomic Number Above 100
Electronic Configurations of Elements and s,p,d &f Blocks
The electronic configuration of an element is determined by the arrangement of electrons in its atomic orbitals. The periodic table provides a systematic arrangement of elements based on their electronic configurations and chemical properties.
Here are some key points regarding electronic configurations and the periodic table:
- An electron in an atom is described by four quantum numbers, with the principal quantum number (n) determining the main energy level or shell.
- The electronic configuration of an element reflects its location in the Periodic Table and the quantum numbers of the last filled orbital.
- Each period in the Periodic Table corresponds to the filling of the next higher principal energy level (n = 1, n = 2, etc.).
- The number of elements in each period is twice the number of available atomic orbitals in the energy level being filled.
- The first period (n = 1) has two elements, hydrogen (1s1) and helium (1s2).
- The second period (n = 2) has eight elements, starting with lithium and ending at neon (2s22p6).
- The third period (n = 3) has eight elements, starting with sodium and ending at argon (3s23p6).
- The fourth period (n = 4) has 18 elements, starting with potassium and ending at krypton (4s24p6), with the filling of 3d orbitals in between.
- The fifth period (n = 5) is similar to the fourth period and contains the 4d transition series.
- The sixth period (n = 6) contains 32 elements and involves the filling of 6s, 4f, 5d, and 6p orbitals.
- The seventh period (n = 7) is similar to the sixth period and includes most of the man-made radioactive elements.
- The lanthanoid series (4f-inner transition series) and actinoid series (5f-inner transition series) are placed separately in the Periodic Table.
- The properties of an element depend on its atomic number rather than its relative atomic mass.
Aufbau Principle
The electronic configurations and types of elements are essential for understanding the periodic classification of elements. The arrangement of electrons in atoms, following the Aufbau (build-up) principle, provides a theoretical basis for organizing elements.
Aufbau Rules:
- Elements in the same vertical column of the Periodic Table form a group or family and exhibit similar chemical behavior.
- This similarity arises because they have the same number and distribution of electrons in their outermost orbitals.
Based on the atomic orbitals being filled with electrons, elements can be classified into four blocks: s-block, p-block, d-block, and f-block.
The s-Block Elements
The s-block elements include Group 1 (alkali metals) and Group 2 (alkaline earth metals). These elements have outermost electronic configurations of ns1 and ns2, respectively
- They are reactive metals with low ionization enthalpies, meaning they readily lose their outermost electrons to form 1+ or 2+ ions.
- As we move down the group, the metallic character and reactivity increase. However, due to their high reactivity, s-block metals are not found in pure form in nature.
- Except for lithium and beryllium, the compounds of s-block elements are predominantly ionic.
The p-Block Elements
The p-block elements consist of elements from Groups 13 to 18, including the noble gases. The outermost electronic configuration of these elements varies from ns2np1 to ns2np6, depending on their position in each period.

- Noble gases have completely filled valence shells (ns2np6) and exhibit very low reactivity.
- Preceding the noble gas family, there are two chemically important groups of nonmetals: the halogens (Group 17) and the chalcogens (Group 16).
- These elements have highly negative electron gain enthalpies and readily gain one or two electrons, respectively, to achieve a stable noble gas configuration.
- The non-metallic character increases from left to right across a period, while the metallic character increases as we move down the group.
The d-Block Elements
The d-block elements, also known as transition elements, occupy Group 3 to 12 in the center of the Periodic Table.

- These elements are characterized by the filling of inner d orbitals with electrons. The general outer electronic configuration for transition elements is (n-1)d1-10ns0-2, except for palladium (Pd) which has the configuration 4d105s0.
- Transition elements are mostly metals that form colored ions, exhibit variable oxidation states (valence), show paramagnetism, and are often used as catalysts.
- However, zinc (Zn), cadmium (Cd), and mercury (Hg), with the electronic configuration (n-1)d10ns2, do not display most of the typical properties of transition elements.
- They act as a bridge between the highly reactive metals of the s-block and the less reactive elements of Groups 13 and 14, earning them the name "Transition Elements."
The f-Block Elements
One important classification is the f-block elements, also known as inner-transition elements, which are located at the bottom of the Periodic Table.

This block is further divided into two series: the lanthanoids and the actinoids.
- Lanthanoids: The lanthanoids span from cerium (Ce) to lutetium (Lu), while the actinoids range from thorium (Th) to lawrencium (Lr). These elements have unique electronic configurations, with the outermost electrons filling the (n-2)f orbitals. This arrangement gives them distinct properties and behaviors. Within each series, the lanthanoids and actinoids share similar characteristics due to the gradual filling of the f-orbitals.
- Actinoids: However, the actinoids possess a more complex chemistry compared to the lanthanoids. This complexity arises from the fact that actinoid elements can exhibit multiple oxidation states, meaning they can lose or gain different numbers of electrons. This versatility in oxidation states leads to a wide range of chemical reactions and compounds. Additionally, it's important to note that actinoid elements are radioactive, making them challenging to study in large quantities.

Metals, Non-metals and Metalloids
Metals
- Metals comprise the majority of elements and are located on the left side of the Periodic Table.
- They are typically solid at room temperature, have high melting and boiling points, and are good conductors of heat and electricity.
- Metals are also known for their malleability, meaning they can be hammered into thin sheets, and ductility, allowing them to be drawn into wires.
Non-metals
- Non Metals, on the other hand, are mainly found on the top right side of the Periodic Table.
- They can exist as either solids or gases at room temperature, have low melting and boiling points (with a few exceptions), and are poor conductors of heat and electricity.
- Non-metallic solids tend to be brittle, meaning they break or shatter easily, and lack the malleability and ductility of metals.
Metalloids
- Metalloids, also known as semi-metals, occupy a diagonal line running from boron (B) to polonium (Po) on the Periodic Table.
- These elements exhibit properties that are intermediate between metals and non-metals.
- They can display characteristics of both groups, such as being semi-conductors of electricity and having varying degrees of malleability and ductility.
- The transition from metallic to non-metallic character is not abrupt but occurs gradually along the diagonal line separating metalloids from the rest of the elements.
- This line represents elements like silicon, germanium, arsenic, antimony, and tellurium.
- They possess properties that are a combination of metals and non-metals, highlighting their semi-metallic nature.
Periodic Trends In Properties of Elements
Periodic Trends in Physical Properties
1. Atomic Radius
The atomic radius refers to the size of an atom, specifically the distance from the nucleus to the outermost electron orbit. It is significant as it allows for comparing atom sizes, shows periodic trends, influences reactivity and bond lengths, and affects physical properties.

Covalent and Metallic Radius
Determining the size of an atom is considerably more challenging than measuring the radius of a ball for a couple of reasons. Firstly, the dimensions of an atom are extremely small, with a radius of approximately 1.2 angstroms (1.2 × 10^–10 meters). Secondly, the lack of a distinct boundary in the electron cloud that envelops the atom makes the precise determination of atomic size difficult.
- An approximation of the atomic size can be derived by considering the distance between atoms when they are in a bonded state. A practical method for estimating the size of a non-metallic atom involves measuring the distance between two atoms forming a single bond in a covalent molecule. From this measurement, the "Covalent Radius" of the element can be calculated. For instance, in the chlorine molecule (Cl2), where the bond distance is 198 picometers (pm), half of this value (99 pm) is considered as the atomic radius of chlorine.
- In the case of metals, we use the term "Metallic Radius," defined as half the distance between the metal cores in the metallic crystal. As an example, the distance between two adjacent copper atoms in solid copper is 256 pm, leading to a metallic radius of copper assigned a value of 128 pm. For simplicity in this context, the term "Atomic Radius" is used to encompass both covalent and metallic radii, depending on whether the element is a non-metal or a metal.

Significance of Atomic Radius
- Size Comparison: The atomic radius allows us to compare the sizes of different atoms. It provides a quantitative measure of the spatial extent of an atom, indicating how much space it occupies. By comparing atomic radii, we can determine which atoms are larger or smaller than others.
- Chemical Reactivity: The atomic radius influences the reactivity of atoms. Atoms with larger radii tend to have more loosely held outermost electrons, making them more likely to participate in chemical reactions. Larger atoms are more prone to losing or gaining electrons to achieve a stable electron configuration, leading to the formation of ions and the establishment of chemical bonds.
- Bond Length: The atomic radius plays a crucial role in determining the length of chemical bonds between atoms. In covalent bonds, the bond length is determined by the sum of the atomic radii of the bonded atoms. Larger atomic radii result in longer bond lengths, while smaller radii lead to shorter bonds.
- Physical Properties: The size of atoms, as indicated by the atomic radius, affects various physical properties such as melting point, boiling point, and density. Larger atoms typically have higher melting and boiling points due to stronger interatomic forces, while smaller atoms tend to have higher densities.
Periodic Trends of Atomic Radius
Atomic Radius Within a Given Period and Group: Within a given period atomic radius decreases from left to right. This is due to the effect of an increase in nuclear charge while the electrons are being added to the same shell. Within a given group atomic radius increases down the group. This is due to the increase in number of shells.

Trends in Atomic Radius
- Atomic Size from Fe to Ni: In the first transition series the atomic size slightly decreases from Sc to Mn because the effect of the effective nuclear charge is stronger than the shielding effect. The atomic size from Fe to Ni remains almost the same because both effects balance each other.
- Atomic Size from Cu to Zn: The atomic size from Cu to Zn slightly increases because shielding effect is more than effective nuclear charge due to d10 structure of Cu and Zn.
- Inner transition elements (Lanthanide Contraction): As we move along the lanthanide series, there is a decrease in atomic as well as ionic radius. The decrease in size is regular in ions but not so regular in atoms. This is called lanthanide contraction.

Atomic Sizes
2. Ionic Radius
Ionic radii can be estimated by measuring the distances between cations and anions in ionic crystals.

- In general, ionic radii follow the same trend as atomic radii.
- Cations are smaller than their parent atoms due to fewer electrons and unchanged nuclear charge.
- Anions are larger than their parent atoms due to increased repulsion among electrons and decreased effective nuclear charge.
- Isoelectronic species, which have the same number of electrons, can have different radii based on their nuclear charges.
- Cations with greater positive charges have smaller radii due to stronger electron-nucleus attraction.
- Anions with greater negative charges have larger radii due to the outweighing repulsion of electrons over nuclear charge.
3. Ionisation Enthalpy
Ionization energy is the energy required to remove an electron from an atom or ion. It represents the minimum energy needed to overcome the attractive forces between the electron and the nucleus. Ionization energy varies depending on the electron being removed and is influenced by factors such as distance from the nucleus and electron configuration.

Factors that influence Ionisation Energy
- Atomic size: the larger the size of the atom, the smaller the I.E. i.e., I.E. µ
- Effective nuclear charge: The greater the effective charge on the nucleus of an atom, the more difficult it would be to remove an electron from the atom because electrostatic force of attraction between the nucleus and the outermost electron increases. So greater energy will be required to remove the electron.
 Ionization energy increases along the period while decreases down the group.
Ionization energy increases along the period while decreases down the group. - Penetration effect of orbitals: The order of energy required to remove an electron from s,p, d-and-f orbitals of a shell is s>p>d>f.
- Shielding or screening effect: The screening effect results in a decrease of the force of attraction between the nucleus and the outermost electron and lesser energy is required to separate the electron. Thus the value of I.P. decreases.
- Stability of half-filled and fully-filled orbitals: According to Hund's rule the stability of half-filled or completely-filled degenerate orbitals is comparatively high. So comparatively more energy is required to separate the electron from such atoms.
 Successive ionisation enthalpies
Successive ionisation enthalpies
4. Electron Gain Enthalpy
Electron Gain Enthalpy refers to the energy change that occurs when an atom or ion gains an electron to form a negatively charged ion. It is also known as electron affinity.
- Definition: Electron Gain Enthalpy is the enthalpy change associated with the process of adding an electron to a gaseous atom or ion to form a gaseous negative ion (anion).
- Sign Convention: Electron Gain Enthalpy can be either exothermic (negative) or endothermic (positive). Exothermic electron gain enthalpy indicates that energy is released during the process, while endothermic electron gain enthalpy indicates energy is absorbed.
Factors affecting the magnitude of electron affinity
- Atomic size – In general electron affinity value decreases with the increasing atomic radius because the electrostatic force of attraction decreases between the electron being added and the atomic nucleus due to the increase in distance between them.
- Effective nuclear charge – The Electron affinity value of the element increases as the effective nuclear charge on the atomic nucleus increases because electrostatic force of attraction between the electron being added and the nucleus increases. As the electrostatic force of attraction increases, amount of energy released is more.
- Screening or Shielding effect – Electron affinity value of the elements decreases with the increasing shielding or screening effect. The shielding effect between the outer electrons and the nucleus increases as the number of electrons increases in the inner shells.
- Stability of half filled and completely filled orbitals – The stability of half filled and completely filled degenerate orbitals of a sub shell is comparatively more, so it is difficult to add electron in such orbitals and lesser energy is released on addition of electron hence the electron affinity value will decrease.
 Electron Affinities (kJ mol–1), M(g) + e– → M(g)– + energy
Electron Affinities (kJ mol–1), M(g) + e– → M(g)– + energy
5. Electronegativity
Electronegativity of elements increases along the period while decreases down the group.
Electronegativity scales
Some arbitrary scales for the quantitative measurement of electronegativities are as under
- Pauling's scale –If xA and xB are the electronegativities of atoms A and B respectively then
0.208 √ΔAB= xA – xB if xA > xB
or ΔAB = 23.06 (xA – xB)2
ΔAB = EA-B(experimental) – EA-B(theoretical)
where EA-B is the energy of A-B bond.
In a purely covalent molecule, AB, the experimental and theoretical values of bond energy A-B are equal.
So ΔAB = 0
or 0=23.06 (xA – xB)2
or xA = xB
In an ionic molecule AB, EA-B(experimental) is more than EA-B(Theoretical).
Pauling assumed the electronegativity value of fluorine to be 4 and calculated the electronegativity values of other elements from this value. - Mulliken's electronegativity: Electronegativitiy = (Electron Afffinity - Ionization Potential)/2
when both are expressed in electron volt - Alfred Rochow’s electronegativity:
If the distance between the circumference of outermost shell and the nucleus is r and the effective nuclear charge Zeff then
Zeff = Z - σ
Z = The actual charge present on the nucleus i.e a number of protons,
σ = Shielding constant
Factors affecting the magnitude of electronegativity
- Atomic radius: As the atomic radius of the element increases the electronegativity value decreases.
- Effective nuclear charge: The electronegativity value increases as the effective nuclear charge on the atomic nucleus increases.
- Oxidation state of the atom: The electronegativity value increases as the oxidation state (i.e. the number of positive charges) of the atom increases.
- Hybridisation state of an atom in a molecule: If the s- character in the hybridisation state of the atom increases, electronegativity also increases.
 Hybridisation and Electronegativity
Hybridisation and Electronegativity
Periodic Trends in Chemical Properties
This section focuses on the periodicity of the valence states shown by elements and the anomalous properties of the second period elements (from lithium to fluorine).
1. Periodicity of Valence or Oxidation States
Valence or oxidation state represents the ability of an atom to combine with other atoms. It is determined by the number of electrons in the outermost shell of an atom. The valency can be calculated based on the following rules:
- If the number of valence electrons is 4 or less, the valency is equal to the number of valence electrons.
- If the number of valence electrons is more than 4, the valency is equal to (8 - number of valence electrons).
(i) In a period, the valency initially increases from 1 to 4 as we move from left to right across the periodic table. After reaching a maximum value of 4, the valency decreases gradually to zero.
(ii) In a group, there is no change in the valency of elements as we move down the group. All elements within a particular group exhibit the same valency.
It is important to note that many elements, especially transition elements and actinoids, can exhibit variable valence, meaning they can have different valence states in different compounds or reactions.
2. Anomalous Properties of Second Period Elements
- The first element of each group (lithium, beryllium, and boron to fluorine) differs from the other members of its group.

Diagonal Relationships
- The first element forms compounds with pronounced covalent character, while the subsequent members predominantly form ionic compounds.
- This behavior shows diagonal relationships, where the first element of a group is more similar to the second element of the following group.
- The anomalous behavior is attributed to the small size, large charge/radius ratio, and high electronegativity of the first member.
- The first member of a group has only four valence orbitals available for bonding, while the subsequent members have nine valence orbitals.
- The first member of p-block elements has a greater ability to form multiple bonds compared to subsequent members of the same group.
3. Periodic Trends and Chemical Reactivity
Periodic trends in atomic and ionic radii, ionization enthalpy, and electron gain enthalpy affect the chemical reactivity of elements.
- Atomic and ionic radii generally decrease from left to right across a period, resulting in higher ionization enthalpies and more negative electron gain enthalpies.
- Elements on the extreme left and right of a period exhibit high chemical reactivity, with the extreme left tending to lose electrons (cation formation) and the extreme right tending to gain electrons (anion formation).
- Metallic character decreases and non-metallic character increases from left to right across a period.
- The reaction of elements with oxygen shows that oxides formed by extreme left elements are basic, those formed by extreme right elements are acidic, and those in the center are amphoteric or neutral.
- Transition metals have smaller changes in atomic radii and intermediate ionization enthalpies, making them less electropositive compared to Group 1 and 2 metals.
- In a group, atomic and ionic radii increase with atomic number, resulting in a gradual decrease in ionization enthalpies and a regular decrease in electron gain enthalpies for main group elements.
- Metallic character increases and non-metallic character decreases down a group, except for transition elements where a reverse trend is observed due to atomic size and ionization enthalpy.
Some Important Questions
Q1: Which of the following statements are not correct?
A. The electron gain enthalpy of F is more negative than that of Cl.
B. Ionization enthalpy decreases in a group of periodic table.
C. The electronegativity of an atom depends upon the atoms bonded to it.
D. Al2O3 and NO are examples of amphoteric oxides.
Choose the most appropriate answer from the options given below :
(a) A, B, C and D
(b) A, C and D Only
(c) A, B and D Only
(d) B and D Only
Ans: (b)
Let's analyze the statements:
A. The electron gain enthalpy of F is more negative than that of Cl.
This statement is incorrect. The electron gain enthalpy of F is less negative than that of Cl due to its small size, which leads to increased electron-electron repulsion when an electron is added.
B. Ionization enthalpy decreases in a group of the periodic table.
This statement is correct. Ionization enthalpy generally decreases down a group of the periodic table due to the increase in atomic size, which results in a weaker attraction between the nucleus and the outermost electrons.
C. The electronegativity of an atom depends upon the atoms bonded to it.
This statement is incorrect. Electronegativity is a property of an atom that depends on the effective nuclear charge and the distance of the outermost electrons from the nucleus. While the difference in electronegativity between atoms in a bond can affect the bond's polarity, the electronegativity itself does not depend on the atoms bonded to it.
D. Al2O3 and NO are examples of amphoteric oxides.
This statement is incorrect. Al2O3 is an example of an amphoteric oxide, meaning it can react with both acids and bases. However, NO is not an amphoteric oxide; it is a neutral oxide.
Hence, the incorrect statements are A, C, and D.
Q2: Group-13 elements react with O2 in amorphous form to form oxides of type M2O3 (M = element). Which among the following is the most basic oxide?
(a) Al2CO3
(b) Tl2O3
(c) B2O3
(d) Ga2O3
Ans: (b)
As electropositive character increases basic character of oxide increases.
Q2: Which of the following elements have half-filled f-orbitals in their ground state?
(Given : atomic number Sm= 62; Eu=63; Tb=65; Gd=64, Pm = 61 )
A. Sm
B. Eu
C. Tb
D. Gd
E. Pm
Choose the correct answer from the options given below :
(a) B and D only
(b) A and B only
(c) C and D only
(d) A and E only
Ans: (a)
Q3: Number of amphoteric compounds among the following is ________.
(A) BeO
(B) BaO
(C) Be(OH)2
(D) Sr(OH)2
Ans: 2
An amphoteric compound is a molecule or ion that can react with both as an acid or as a base.
BeO = Amphoteric
BaO = Basic
Be(OH)2 = Amphoteric
Sr(OH)2 = Basic
Both beryllium compound BeO and Be(OH)2 are amphoteric in nature while compound BaO and Sr(OH)2 are basic in nature, they form alkaline solution in H2O.
FAQs on Classification of Elements & Periodicity in Properties Class 11 Notes Chemistry Chapter 3
| 1. Why is it important to classify elements in chemistry? |  |
| 2. What are the key differences between the modern periodic table and earlier classifications? |  |
| 3. How are elements with atomic numbers greater than 100 named? |  |
| 4. What are the electronic configurations of s, p, d, and f block elements? |  |
| 5. What are the periodic trends in the properties of elements? |  |

|
Explore Courses for NEET exam
|

|