Aromatic Hydrocarbons: Nomenclature, Properties, Reactions, Uses & Polycyclic Aromatic Hydrocarbons | Chemistry Class 11 - NEET PDF Download
What are Aromatic Hydrocarbons?
Aromatic Hydrocarbons are circularly structured organic compounds that contain sigma bonds along with delocalized pi electrons. They are also referred to as arenes or aryl hydrocarbons.
Aromatic hydrocarbon, commonly known as arenes are hydrocarbons containing sigma bonds and delocalized pi electrons between the carbon atoms in a ring. For example, benzene. They are known as aromatic due to their pleasant smell.

IUPAC Nomenclature of aromatic hydrocarbons
Earlier, most of the compounds with the same structural formula were known by different names depending on the regions where they were synthesized. This naming system was very trivial since it raised a lot of confusion. Finally, a common naming system enlisting standard rules was set up by IUPAC (International Union for Pure and Applied Chemistry) for the naming of compounds. This method of naming is IUPAC naming or IUPAC nomenclature. IUPAC nomenclature of aromatic hydrocarbons is explained below:
1. According to IUPAC nomenclature of substituted aromatic compounds, the substituent name is placed as a prefix to the name of aromatic compounds. For example, a benzene ring attached to one-nitro group is named as nitrobenzene.
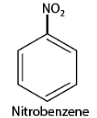
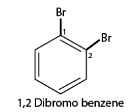
2. When more than one similar substituent group are present in the ring, they are labeled with the Greek numerical prefixes such as di, tri, tetra to denote the number of similar substituent groups attached to the ring. If two bromo- groups are attached to the adjacent carbon atoms of the benzene ring, it is named as 1,2-dibromobenzene.
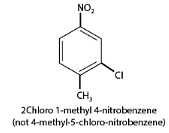
3. When different substituted groups are attached to the aromatic compounds, the substituent of base compound is assigned number one and then the direction of numbering is chosen such that the next substituent gets the lowest number. Substituents are named in alphabetical order. For example: when chloro and nitro groups are attached to the benzene ring, we first locate the chloro group then nitro groups.
4. In case of multiple substituted aromatic compounds, sometimes terms like ortho (o), meta (m) and para (p) are also used as prefixes to indicate the relative positions 1,2- ;1,3- and 1,4- respectively. For example, 1,2 di-bromo-benzene can be named as o-di-bromo-benzene.
5. When an alkane with a functional group is attached to an aromatic compound, the aromatic compound is considered as a substituent, instead of a parent. For example: when a benzene ring is attached to an alkane with a functional group, it is considered as a substituent named as phenyl, denoted by Ph-.
[Intext Question]
Properties of Aromatic Hydrocarbons
The first compound that was categorized as an aromatic hydrocarbon was benzene.
It is also the most complex aryl hydrocarbon. Each carbon atom belonging to the benzene ring has two carbon-carbon sigma bonds, one carbon-hydrogen sigma bond, and one double bond with a neighbouring carbon in which the pi electron is delocalized.
This delocalization of pi electrons in the benzene molecule is represented by a circle inside the hexagon. The bond order of all carbon-carbon bonds in this molecule is considered to be 1.5 and this equivalency can be explained with the help of the resonance structures of benzene.
Some general properties of aromatic hydrocarbons have been listed below:
- These compounds exhibit aromaticity (additional stability granted by resonance)
- The ratio of carbon atoms to hydrogen atoms is relatively high in these types of molecules.
- When burnt, the aromatic hydrocarbons display a strong and sooty flame which is yellow.
- These compounds generally undergo electrophilic substitutions and nucleophilic aromatic substitution reactions.
- It can be noted that these compounds can be either monocyclic or polycyclic.
Reactions of Aromatic Hydrocarbons
Many organic chemical reactions involve the use of aromatic hydrocarbons as the primary reactant. Some such reactions are listed in this subsection along with a brief description of each of these reactions.
1. Aromatic Substitution Reactions
These reactions involve the replacement of one substituent on the ring of an aromatic hydrocarbon, commonly a hydrogen atom, by a different substituent group.
The common types of aromatic substitution reactions include:
- Nucleophilic aromatic substitution reactions
- Electrophilic aromatic substitution reactions
- Radical nucleophilic aromatic substitution reactions
An example of an aromatic substitution reaction is the electrophilic substitution observed in the nitration reaction of salicylic acid.
2. Coupling Reactions
In these types of reactions, the coupling of two fragments that have a radical nature is achieved with the help of a metal catalyst. When aromatic hydrocarbons undergo coupling reactions, the following type of bonds can be formed.
- Carbon-carbon bonds can be formed from the coupling reactions of arenes and products such as vinyl arenes, alkyl arenes, etc. are formed.
- The formation of carbon-oxygen bonds can occur in these reactions, forming aryloxy compounds.
- Carbon-nitrogen bonds can form in coupling reactions, giving products such as aniline.
An example of a coupling reaction involving aromatic hydrocarbons can be observed in the arylation of perfluorobenzenes, as illustrated below.
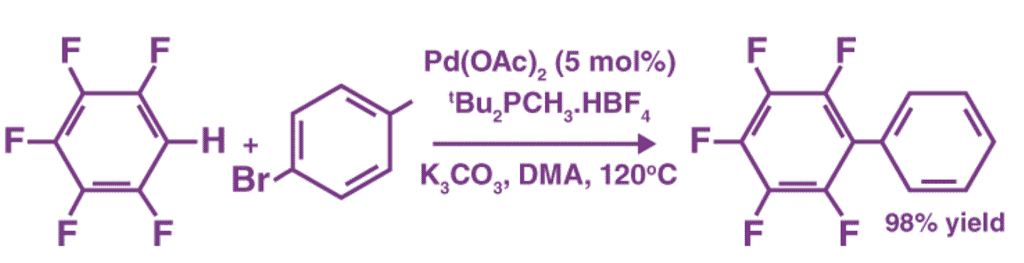
The catalyst used in this reaction is Palladium(II) acetate. It can also be noted that DMA is the abbreviation of Dimethylacetamide.
3. Hydrogenation Reactions
- The hydrogenation reactions involving arenes generally lead to the formation of saturated rings. An example of such a reaction is the reduction of 1-naphthol into a mixture containing different isomers of decalin-ol.
- Another example of such a reaction is the hydrogenation reaction of resorcinol with the help of spongy nickel (also referred to as Raney nickel) and aqueous NaOH. This reaction proceeds via the formation of an enolate, and the successive alkylation of this enolate (with methyl iodide) to yield 2-methyl-1,3-cyclohexanedione.
Uses of Aromatic Hydrocarbons
The use of aromatic hydrocarbons is common in both biological and synthetic processes.
- The green pigment found in plants, more commonly known as chlorophyll, consists of aromatic hydrocarbons and is very important in the process of food production in plants.
- The nucleic acids and amino acids in the human body also consist of these aromatic hydrocarbons.
- Methylbenzene which is an aromatic hydrocarbon is used as a solvent in model glues
- Naphthalene is an important item in the production of mothballs
- For the synthesis of drugs, dyes, and explosives, an aryl hydrocarbon known as Phenanthrene is used
- Trinitrotoluene or TNT is a very important aromatic hydrocarbon which is widely used for explosive purposes.
- Plastic industry and petrochemical industries make use of aromatic hydrocarbons extensively.
Polycyclic Aromatic Hydrocarbons
- These are the hydrocarbons which comprise aromatic rings in fused form. These are found in coal, tar, oil and some cooked foods such as smoked fish, burnt toast, etc.
- One common example of these polycyclic hydrocarbons is naphthalene. These compounds are said to be pollutants.
- Some examples of aromatic hydrocarbons are Methylbenzene, Naphthalene, Phenanthrene, Trinitrotoluene, and o-dihydroxybenzene.
|
129 videos|244 docs|88 tests
|
FAQs on Aromatic Hydrocarbons: Nomenclature, Properties, Reactions, Uses & Polycyclic Aromatic Hydrocarbons - Chemistry Class 11 - NEET
| 1. What is the IUPAC nomenclature for aromatic hydrocarbons? |  |
| 2. What are some properties of aromatic hydrocarbons? |  |
| 3. What are some common reactions of aromatic hydrocarbons? |  |
| 4. What are some common uses of aromatic hydrocarbons? |  |
| 5. What are polycyclic aromatic hydrocarbons (PAHs) and how are they different from aromatic hydrocarbons? |  |

|
Explore Courses for NEET exam
|

|

















