Solutions of Chemical Reactions and Equations (Page No - 50) - Chemistry Lakhmir Singh, Class 10 | Extra Documents, Videos & Tests for Class 10 PDF Download
Question 64:
Two metals X and Y form the salts XSO4 and Y2SO4, respectively. The solution of salt XSO4 is blue in colour whereas that of Y2SO4 is colourless. When barium chloride solution is added to XSO4 solution, then a white precipitate Z is formed along with a salt which turns the solution green. And when barium chloride solution is added to Y2SO4 solution, then the same white precipitate Z is formed along with colourless common salt solution.
(a) What could the metals X and Y be ?
(b) Write the name and formula of salt XSO4.
(c) Write the name and formula of salt Y2SO4.
(d) What is the name and formula of white precipitate Z ?
(e) Write the name and formula of the salt which turns the solution green in the first case.
Solution :
(a) Metal X : Copper; Metal Y : Sodium
(b) Copper sulphate, CuSO4
(c) Sodium sulphate, Na2SO4
(d) Barium sulphate, BaSO4
(e) Copper chloride, CuCl2
Question 65:
A red-brown metal X forms a salt XSO4. When hydrogen sulphide gas is passed through an aqueous solution of XSO4, then a black precipitate of XS is formed alongwith sulphuric acid solution.
(a) What could the salt XSO4 be ?
(b) What is the colour of salt XSO4 ?
(c) Name the black precipitate XS.
(d) By using the formula of the salt obtained in (a) above, write an equation of the reaction which takes place when hydrogen sulphide gas is passed through its aqueous solution.
(e) What type of chemical reaction takes place in this case ?
Solution :
(a) Copper sulphate.
(b) Blue colour.
(c) Copper sulphide.
(d) CuSO4 (aq) + H2S (g)—–>CuS (s) + H2SO4 (aq)
(e) Double displacement reaction.
Question 66:
When a strip of red-brown metal X is placed in a colourless salt solution YNO3 then metal Y is set free and a blue coloured salt solution X(NO3)2 is formed. The liberated metal Y forms a shining white deposit on the strip of metal X.
(a) What do you think metal X is ?
(b) Name the salt YNO3.
(c) What could be metal Y ?
(d) Name the salt X(NO3)2.
(e) What type of reaction takes place between metal X and salt solution YNO3 ?
Solution :
(a) Copper.
(b) Silver nitrate.
(c) Silver.
(d) Copper nitrate.
(e) Displacement reaction.
Question 67:
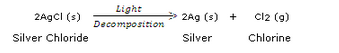
A metal salt MX when exposed to light splits up to form metal M and a gas X2. Metal M is used in making ornaments whereas gas X2 is used in making bleaching powder. The salt MX is itself used in black and white photography.
(a) What do you think metal M is ?
(b) What could be gas X2 ?
(c) Name the metal salt MX.
(d) Name any two salt solutions which on mixing together can produce a precipitate of salt MX.
(e) What type of chemical reaction takes place when salt MX is exposed to light ? Write the equation of the reaction.
Solution :
(a) Silver.
(b) Chlorine.
(c) Silver chloride.
(d) Silver nitrate and Sodium chloride.
(e) Decomposition reaction;
|
5 videos|292 docs|59 tests
|
FAQs on Solutions of Chemical Reactions and Equations (Page No - 50) - Chemistry Lakhmir Singh, Class 10 - Extra Documents, Videos & Tests for Class 10
| 1. What are chemical reactions and equations? |  |
| 2. How can we balance a chemical equation? |  |
| 3. What is the importance of balancing chemical equations? |  |
| 4. What are the different types of chemical reactions? |  |
| 5. How do chemical reactions and equations relate to everyday life? |  |
|
5 videos|292 docs|59 tests
|

|
Explore Courses for Class 10 exam
|

|

















