Solutions of Metals and Non-metals (Page No - 169) - Chemistry Lakhmir Singh, Class 10 | Extra Documents, Videos & Tests for Class 10 PDF Download
Question 35:
Describe how sodium and chlorine atoms are changed into ions when they react with each other to form sodium chloride, NaCl. What is the name given to this type of bonding ? (At. No of sodium = 11; At. No. of chlorine = 17)
Solution :
The atomic number of sodium is 11, so its electronic configuration is 2,8,1. Sodium atom has only 1 electron in its outermost shell. So, the sodium atom donates one electron (to a chlorine atom) and forms a sodium ion, Na+ . The atomic number of chlorine is 17, so its electronic configuration is 2,8,7. Chlorine atom has 7 electrons in its outermost shell and needs 1 more electron to achieve the stable 8-electron inert gas configuration. So, a chlorine atom takes one electron (from the sodium atom) and forms a negatively charged chloride ion, Cl- This type of bonding is called ionic bonding.
Question 36:
What is the difference between a cation and an anion ? How are they formed ? Give the names and symbols of one cation and one anion.
Solution :
A positively charged ion is known as cation. A cation is formed by the loss of one or more electrons by an atom. For example: sodium loses 1 electron to form a sodium ion, Na + , which is a cation.
A negatively charged ion is known as anion. An anion is formed by the gain of one or more electrons by an atom. For example: A chlorine atom gains (accepts) 1 electron to form a chloride ion, Cl – , which is an anion.
Question 37:
Using electron-dot diagrams which show only the outermost shell electrons, show how a molecule of nitrogen, N2, is formed from two nitrogen atoms. What name is given to this type of bonding ? (Atomic number of nitrogen is 7)
Solution :
Since nitrogen has 5 electrons in its outermost shell so, to achieve the 8-electron structure of an inert gas, it needs 3 more electrons and hence combines with another nitrogen atom to form a molecule of nitrogen gas.
This type of bonding is called covalent bonding.
Question 38:
Draw the electron-dot structures of the following compounds and state the type of bonding in each case :
(i) CO2 (ii) MgO (iii) H,O (iv) HCl (v) MgCl2
Solution :


Question 39:
Using electron-dot diagrams which show only the outermost shell electrons, show how a molecule of oxygen, O2, is formed from two oxygen atoms. What name is given to this type of bonding ? (At. No. of oxygen = 8)
Solution :
Since an oxygen atom has 6 electrons in its outermost shell so, it needs 2 more electrons to achieve the stable 8-electron inert gas configuration. Hence, it combines with another oxygen atom and forms a molecule of oxygen.
This type of bonding is called a double covalent bond.
Question 40:
Draw the electron-dot structures of the following compounds and state the type of bonding in each case :
(i) KCl (ii) NH3 (iii) CaO (iv) N2 (iv) CaCl2
Solution :


Question 41:
Explain why, a salt which does not conduct electricity in the solid state becomes a good conductor in molten state.
Solution :
Although solid ionic compounds are made up of ions but they do not conduct electricity in solid state. This is because in the solid ionic compound, the ions are held together in fixed positions by strong electrostatic forces and cannot move freely. However, when we dissolve the ionic solid in water or melt it, the crystal structure is broken down and ions become free to move and conduct electricity. Thus, an aqueous solution of an ionic compound conducts electricity because there are plenty of free ions in the solution which are able to conduct electric current.
Question 42:
(a) Write down the electronic configuration of (i) sodium atom, and (ii) chlorine atom.
(b) How many electrons are there in the outermost shell of (i) a sodium atom, and (ii) a chlorine atom ?
(c) Show the formation of NaCl from sodium and chlorine atoms by the transfer of electron(s).
(d) Why has sodium chloride a high melting point ?
(e) Name the anode and the cathode used in the electrolytic refining of impure copper metal.
Solution :
(a) (i) Sodium – 2, 8, 1 (ii) Chlorine – 2, 8, 7
(b) (i) Sodium = 1 (ii) Chlorine = 7
(d) Sodium chloride has a high melting point because it is an ionic compound and these compounds are made of up of positive and negative ions. There is a strong force of attraction between the oppositely charged ions, so, a lot of heat energy is required to break this force of attraction and melt or boil the ionic compound.
(e) Anode: Thick block of impure copper metal; Cathode: Thin strip of p ure copper metal
Question 43:
(a) Write the electron arrangement in (i) a magnesium atom, and (ii) an oxygen atom.
(b) How many electrons are there in the valence shell of (i) a magnesium atom, and (ii) an oxygen atom ?
(c) Show on a diagram the transfer of electrons between the atoms in the formation of MgO.
(d) Name the solvent in which ionic compounds are generally soluble.
(e) Why are aqueous solutions of ionic compounds able to conduct electricity ?
Solution :
(a) (i) Magnesium -2, 8, 2
(ii) Oxygen – 2, 6
(b) (i) Magnesium = 2
(ii) Oxygen = 6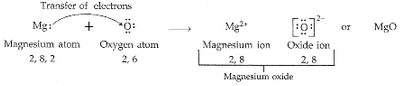
(d) Water .
(e) An aqueous solution of an ionic compound conducts electricity because there are plenty of free ions in the solution which are able to conduct electric current.
Question 44:
(a) What is the electronic configuration of (i) a sodium atom, and (ii) an oxygen atom ?
(b) What is the number of outermost electrons in (i) a sodium atom, and (ii) an oxygen atom ?
(c) Show the formation of Na20 by the transfer of electrons between the combining atoms.
(d) Why are ionic compounds usually hard ?
(e) How is it that ionic compounds in the solid state do not conduct electricity but they do so when in molten state ?
Solution :
(a) (i) 2,8,1 (ii) 2,6
(b) (i) 1 (ii) 6
(c) 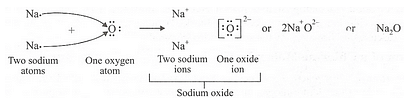
(d)I onic compounds are usually hard because their oppositely charged ions attract one another strongly and form a regular crystal structure.
(e) Although solid ionic compounds are made up of ions but they do not conduct electricity in solid state. This is because in the solid ionic compound the ions are held together in fixed positions by strong electrostatic forces and cannot move freely. However, when we dissolve the ionic solid in water or melt it, the crystal structure is broken down and ions become free to move and conduct electricity. Thus, an aqueous solution of an ionic compound conducts electricity because there are plenty of free ions in the solution which are able to conduct electric current.
Question 45:
(a) Write down the electron arrangement in (i) a magnesium atom, and (ii) a chlorine atom.
(b) How many electrons are there in the valence shell of (i) a magnesium atom, and (ii) a chlorine atom ?
(c) Show the formation of magnesium chloride from magnesium and chlorine by the transfer of electrons.
(d) State whether magnesium chloride will conduct electricity or not. Give reason for your answer.
(e) Why are covalent compounds generally poor conductors of electricity ?
Solution :
(a)(i) Magnesium: 2, 8, 2 (ii) Chlorine: 2, 8, 7
(b)(i) 2 (ii) 7
(c) 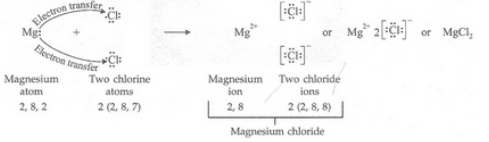
(d)Magnesium chloride will conduct electricity because it is an ionic compound .(e) Covalent compounds are generally poor conductors of electricity b ecause they do not contain ions.
|
5 videos|292 docs|59 tests
|
FAQs on Solutions of Metals and Non-metals (Page No - 169) - Chemistry Lakhmir Singh, Class 10 - Extra Documents, Videos & Tests for Class 10
| 1. What are metals and non-metals? |  |
| 2. Give examples of metals and non-metals. |  |
| 3. How do metals and non-metals differ in terms of their physical properties? |  |
| 4. What are the chemical properties of metals and non-metals? |  |
| 5. How are metals and non-metals used in everyday life? |  |
|
5 videos|292 docs|59 tests
|

|
Explore Courses for Class 10 exam
|

|

















