Solutions of Carbon And Its Compounds (Page No - 266) - Chemistry Lakhmir Singh, Class 10 | Extra Documents, Videos & Tests for Class 10 PDF Download
Question 66:
A neutral organic compound X of molecular formula C2H6O on oxidation with acidified potassium dichromate gives an acidic compound Y. Compound X reacts with Y on warming in the presence of cone. H2S04 to give a sweet smelling substance Z. What are X, Y and Z ?
Solution :
X is ethanol
Y is ethanoic acid
Z is ethyl ethanoate
Ethanol reacts with ethanoic acid to form ethyl ethanoate ester.
Question 67:
Consider the following organic compounds :
HCHO, C2H5OH, C2H6, CH3COOH, C2H5C1
Choose two compounds which can react in the presence of cone. H2S04 to form an ester. Give the name and formula of the ester formed.
Solution :
C2H5OH and CH3COOH react in the presence of conc. H2SO4 to form an ester. Ethyl ethanoate, CH3COOC2H5 is formed in the reaction.
Question 68:
A neutral organic compound is warmed with some ethanoic acid and a little of cone. H2S04. Vapours having sweet smell (fruity smell) are evolved. What type of functional group is present in this organic compound ? The structural formula of an ester is :
Solution :
Alcohol group, -OH. Acids react with alcohols to form sweet smelling esters.
Question 69:
The structural formula of an ester is :
Write the formula of the acid and the alcohol from which it is formed.
Solution :
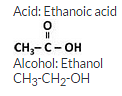
Question 70:
Consider the following organic compounds :
CH3OH, C2H5OH, CH3COCH3, CH3COOH, C2H5COOH, C4H9COOC2H5, CH4, C2H6, CH3CHO, HCHO Out of these compounds :
(a) Which compound is most likely to be sweet-smelling ?
(b) Which compound on treatment with cone. H2S04 at 170°C forms an alkene ?
(c) Which compound on repeated chlorination forms chloroform ?
(d) Which compound is added to alcohol to denature it ?
(e) Which compound is a constituent of vinegar ?
(f) Which compound is used to sterilise wounds and syringes ?
Solution :
(a) C4H9COOC2H5; Ester
(b) C2H5OH; Alcohol forms ethene, C2H4
(c) CH4; Methane
(d) CH3OH; Methanol
(e) CH3COOH; Acetic acid
(f) C2H5OH; Ethanol
Question 71:
An organic acid X is a liquid, Which often freezes during winter time in cold countries, having the molecular formula C2H402. On warming it with methanol in the presence of a few drops of concentrated sulphuric acid, a compound Y with a sweet smell is formed.
(a) Identify X and Y. Also write their formulae showing the functional group present in them.
(b) Write a chemical equation for the reaction involved.
Solution :
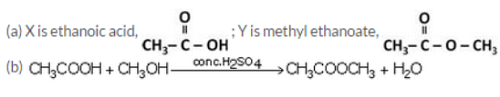
Question 72:
An organic compound A having the molecular formula C3H8O is a liquid at room temperature. The organic liquid A reacts with sodium metal to evolve a gas which burns causing a little explosion. When the organic liquid A is heated with concentrated sulphuric acid at 170°C, it forms a compound B which decolourises bromine water. The compound B adds on one molecule of hydrogen in the presence of Ni as catalyst to form compound C which gives substitution reactions with chlorine.
(a) What is compound A ?
(b) What is compound B ?
(c) What type of reaction occurs when A is converted into B ?
(d) What is compound C ?
(e) What type of reaction takes place when B is converted into C ?
Solution :
(a) A is propanol, CH3-CH2-CH2OH
(b) B is propene, CH3CH=CH2
(c) Dehydration reaction
(d) C is propane, CH3CH2-CH3
(e) Addition reaction
|
5 videos|292 docs|59 tests
|
FAQs on Solutions of Carbon And Its Compounds (Page No - 266) - Chemistry Lakhmir Singh, Class 10 - Extra Documents, Videos & Tests for Class 10
| 1. What is the importance of carbon in compounds? |  |
| 2. What are some common examples of carbon compounds? |  |
| 3. How does carbon form covalent bonds with other elements? |  |
| 4. What is the significance of carbon compounds in everyday life? |  |
| 5. How do carbon compounds contribute to climate change? |  |
|
5 videos|292 docs|59 tests
|

|
Explore Courses for Class 10 exam
|

|

















