Solutions of Periodic Classification Of Elements (Page No - 284) - Chemistry Lakhmir Singh, Class 10 | Extra Documents, Videos & Tests for Class 10 PDF Download
Question 42:
The atomic masses of three elements X, Y and Z having similar chemical properties are 7, 23 and 39 respectively.
(a) Calculate the average atomic mass of elements X and Z.
(b) How does the average atomic mass of elements X and Z compare with the atomic mass of element Y ?
(c) Which law of classification of elements is illustrated by this example ?
(d) What could the elements X, Y and Z be ?
(e) Give another example of a set of elements which can be classified according to this law.
Solution :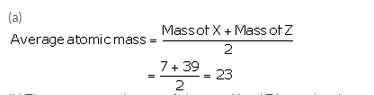
(b) The average atomic mass of elements X and Z is equal to the atomic mass of element Y
(c) Dobereiner’s law of triads.
(d) X is lithium, Y is sodium and Z is potassium .
(e) Chlorine, Bromine, Iodine .
Question 43:
In the following set of elements, one element does not belong to the set. Select this element and explain why it does not belong :
Calcium, Magnesium, Sodium, Beryllium
Solution :
Sodium does not belong to the set. This is because all other elements belong to group 2 but sodium belongs to group 1 .
Question 44:
In the following set of elements, one element does not belong to the set. Select this element and state why it does not belong :
Oxygen, Nitrogen, Carbon, Chlorine, Fluorine
Solution :
Chlorine does not belong to the set. This is because all other elements belong to 2nd period whereas chlorine belongs to 3rd period.
Question 45:
Can the following groups of elements be classified as Dobereiner’s triads ?
(a) Na, Si, Cl (b) Be, Mg, Ca
Give reason for your answer.
(Atomic masses : Be 9 ; Na 23 ; Mg 24 ; Si 28 ; Cl 35.5 ; Ca 40)
Solution :
(a) No. This is because the elements Na, Si and Cl do not have similar properties even though the atomic mass of middle element Si is almost equal to the average atomic mass of first element Na and third element Cl.
(b) Yes. This is because the elements Be, Mg and Ca have similar properties and the atomic mass of middle element Mg is almost equal to the average atomic mass of first element Be and third element Ca
Question 46:
Consider the following elements :
Na, Ca, Al, K, Mg, Li
(a) Which of these elements belong to the same period of the periodic table ?
(b) Which of these elements belong to the same group of the periodic table ?
Solution :
(a) Same period (Third period): Na, Mg, Al.
(b) Same group (First group): Li, Na, K .
Question 47:
Which element has :
(a) two shells, both of which are completely filled with electrons ?
(b) the electronic configuration 2, 8, 2 ?
(c) a total of three shells, with four electrons in its valence shell ?
(d) a total of two shells, with three electrons in its valence shell ?
(e) twice as many electrons in its second shell as its first shell ?
Solution :
(a) Neon (2, 8).
(b) Magnesium .
(c) Silicon (2, 8, 4).
(d) Boron (2, 3) .
(e) Carbon (2, 4)
|
5 videos|292 docs|59 tests
|
FAQs on Solutions of Periodic Classification Of Elements (Page No - 284) - Chemistry Lakhmir Singh, Class 10 - Extra Documents, Videos & Tests for Class 10
| 1. What is periodic classification of elements? |  |
| 2. How are elements arranged in the periodic table? |  |
| 3. What are the periods and groups in the periodic table? |  |
| 4. Why is periodic classification of elements important? |  |
| 5. What are the trends in the periodic table? |  |
|
5 videos|292 docs|59 tests
|

|
Explore Courses for Class 10 exam
|

|












