Solutions of Periodic Classification Of Elements (Page No - 308) - Chemistry Lakhmir Singh, Class 10 | Extra Documents, Videos & Tests for Class 10 PDF Download
Question 74:
The following diagram shows a part of the periodic table containing first three periods in which five elements have been represented by the letters a, b, c, d and e (which are not their chemical symbols):
(i) Select the letter which represents an alkali metal.
(ii) Select the letter which represents a noble gas.
(iii) Select the letter which represents a halogen.
(iv) What type of bond is formed between a and e?
(v) What type of bond is formed between d and e ?
Solution :
(i) d.
(ii)c.
(iii) e.
(iv) Covalent bond .
(v) Ionic bond .
Question 75:
The elements A, B and C belong to groups 1, 14 and 17 respectively of the periodic table.
(a) Which two elements will form a covalent compound ?
(b) Which two elements will form an ionic compound ?
Solution :
(a) B and C.
(b) A and C .
Question 76:
Find the neutral atom in the periodic table which has the same number of electrons as K+ and Cl . What is this number ?
Solution :
Argon atom, 18 electrons .
Question 77:
Atoms of eight elements A, B, C, D, E, F, G and H have the same number of electron shells but different
number of electrons in their outermost shells. It was found that elements A and G combine to form an ionic compound. This ionic compound is added in a small amount to almost all vegetables and dishes during
cooking. Oxides of elements A and B are basic in nature while those of elements E and F are acidic. The
oxide of element D is, however, almost neutral. Based on the above information, answer the following questions :
(a) To which group or period of the periodic table do these elements belong ?
(b) What would be the nature of compound formed by a combination of elements B and F ?
(c) Which two of these elements could definitely be metals ?
(d) Which one of the eight elements is most likely to be found in gaseous state at room temperature ?
(e) If the number of electrons in the outermost shell of elements C and G be 3 and 7 respectively, write the formula of the compound formed by the combination of C and G.
Solution :
(a) 3rd period.
(b) Ionic compound .
(c) A and B.
(d) H.
(e) CG3 .
Question 78:
Write the names and symbols of two very reactive metals belonging to group 1 of the periodic table. Explain by drawing electronic structure, how either one of the two metals reacts with a halogen. With which name is the bond formed between these elements known and what is the class of the compound so formed known ? State any four physical properties of such compounds.
Solution :
Sodium (Na) and Potassium (K);
Sodium (Na) is a metal. So, sodium readily reacts with a halogen like chlorine (Cl) to form an ionic chloride called sodium chloride. This is illustrated below:
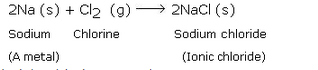
Ionic bond; Ionic compounds .
Physical properties of ionic compounds:
(i) Ionic comp ounds are usually hard, brittle.
(ii) They conduct electricity when molten or dissolved.
(iii) They have high melting and boiling points.
(iv) Most are soluble in polar solvents such as water.
Question 79:
The non-metal A is an important constituent of our food and most of the fuels around us. A forms two oxides B and C. The oxide B is poisonous whereas oxide C causes global warming.
(a) Identify A, B and C.
(b) To which group of periodic table does A belong ?
(c) Name another element which is placed in the same group as A.
Solution :
(a) A is carbon (C); B is carbon monoxide (CO) ; C is carbon dioxide (CO 2 ).
(b) 14 th group .
(c) Silicon (Si) .
Question 80:
A non-metal X which is the largest constituent of air combines with hydrogen when heated in the presence of iron as catalyst to form a gas Y. When gas Y is treated with sulphuric acid, it forms a compound Z which is used as a chemical fertiliser.
(a) What are X, Y and Z ?
(b) To which group of periodic table does X belong ?
(c) Name the period of periodic table in which X is placed.
(d) Which element is placed just before X in the period ?
(e) Which element is placed just after X in the period ?
Solution :
(a) X is nitrogen gas, N2 ;
Y is ammonia gas, NH3
and Z is ammonium sulphate, (NH 4)2SO4.
(b) 15 th group .
(c) 2 nd period.
(d) Carbon, C .
(e) Oxygen, O.
|
5 videos|292 docs|59 tests
|
FAQs on Solutions of Periodic Classification Of Elements (Page No - 308) - Chemistry Lakhmir Singh, Class 10 - Extra Documents, Videos & Tests for Class 10
| 1. What is periodic classification of elements? |  |
| 2. Why is periodic classification of elements important? |  |
| 3. How are elements arranged in the periodic table? |  |
| 4. What are the periods and groups in the periodic table? |  |
| 5. How does the periodic classification of elements help in predicting properties? |  |
|
5 videos|292 docs|59 tests
|

|
Explore Courses for Class 10 exam
|

|

















