Acid Rain - Class 8 PDF Download
Ref: https://edurev.in/question/682395/give-10-harmful-effects-of-the-acid-rain-Related-What-is-Meant-by-Acid-Rain-Pollution-of-Air-and-W
What is Acid Rain?
Acid Rain as the name suggests can be said to be the precipitation of acid in the form of rain in the simplest manner. When atmospheric pollutants like oxides of nitrogen and sulphur react with rainwater and come down with the rain, then this results in Acid Rain.
What Causes Acid Rain?
The causes of acid rain are the Sulfur and Nitrogen particles which get mixed with the wet components of rain. Sulfur and Nitrogen particles which get mixed with water are found in two ways either man-made i.e as the emissions given out from industries or by natural causes like how a lightning strike in the atmosphere releases nitrogen ions and sulphur is released from volcanic eruptions.
The regular clean rain we experience, even though it is not clean i.e water and carbon dioxide react together to form weak carbonic acid which essentially by itself is not extremely harmful. The reaction occurring is :
H2O (l) + CO2 (g) ⇌ H2CO3 (aq)
The pH value of regular rainwater is around 5.7, giving it an acidic nature. The oxides of nitrogen and sulphur are blown away by the wind along with the dust particles. They settle on the earth’s surface after coming down in the form of precipitation. Acid rain is essentially a byproduct of human activities which emit oxides of nitrogen and sulphur in the atmosphere. Example – burning of fossil fuels, unethical waste emission disposal techniques.

Formation of Acid Rain
Sulphur dioxide and nitrogen dioxide undergo oxidation and then they react with water resulting in the formation of sulphuric acid and nitric acid respectively. The following reaction will clarify the acid formation reaction:
2SO2 (g) + O2 (g) + 2H2O (l) → 2H2SO4 (aq)
4NO2 (g) + O2 (g) + 2H2O (l) → 4HNO3 (aq)
Effects of acid rain:
- Acid rain is very harmful to agriculture, plants, and animals. It washes away all nutrients which are required for the growth and survival of plants. Acid rain affects agriculture by the way how it alters the composition of the soil.

The effect of Acid Rain on a forest
- It causes respiratory issues in animals and humans.
- When acid rain falls down and flows into the rivers and ponds it affects the aquatic ecosystem. As it alters the chemical composition of the water, to a form which is actually harmful to the aquatic ecosystem to survive and causes water pollution.
- Acid rain also causes the corrosion of water pipes. Which further results in leaching of heavy metals such as iron, lead and copper into drinking water.
- It damages the buildings and monuments made up of stones and metals.

A statue which has been withered away due to Acid Rain
Real life examples:
- Taj Mahal, one of the 7 wonders of the world, is largely affected by acid rain. The city of Agra has many industries which emit the oxides of sulphur and nitrogen in the atmosphere. People continue to use low-quality coal and firewood as domestic fuel, adding to this problem. Acid rain has the following reaction with the marble (calcium carbonate):
CaCO3 + H2SO4 → CaSO4 + H2O + CO2
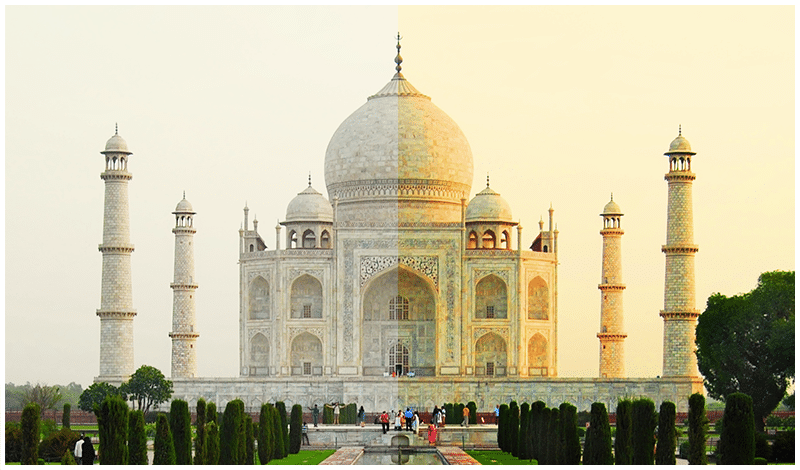
Before and After effects of acid rain on the Taj Mahal
The formation of calcium sulphate results in the corrosion of this beautiful monument.
- Statue of Liberty which is made of copper has also been damaged by the cumulative action of acid rain & oxidation for over 30 years and is, therefore, becoming green in colour.

Before and After images of the impact of Acid Rain on the Statue Of Liberty
How to prevent Acid Rain?
The only precaution that we can take against acid rain is having a check at the emission of oxides of nitrogen and sulphur. We have so far seen the details of acid rain and its harmful effect on animals, plants and the monuments. Being responsible citizens, one should be aware of the harmful effects they cause and of the industries which give out nitrogen and sulphur compound wastes unethically.
FAQs on Acid Rain - Class 8
| 1. What is acid rain? |  |
| 2. What are the main causes of acid rain? |  |
| 3. How does acid rain affect the environment? |  |
| 4. What are the impacts of acid rain on human health? |  |
| 5. How can we reduce acid rain? |  |



















