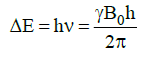Nuclear Magnetic Resonance (NMR) Spectroscopy | Organic Chemistry PDF Download
NMR is the most powerful tool available for organic structure determination. It is used to study a wide variety of nuclei: 1H, 13C, 15N, 19F, 31P.
Nuclear Spin
- A nucleus with an odd atomic number or an odd mass number has a nuclear spin.
- The spinning charged nucleus generates a magnetic field.
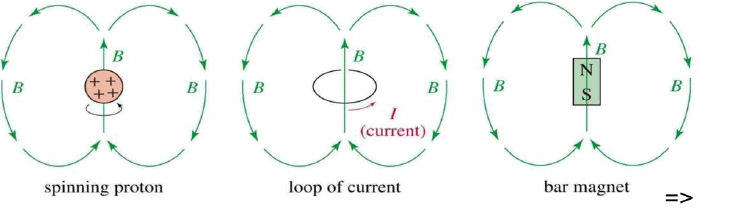
- Number of spin states for that nuclei is defined by (2I+1),with integral differences ranging from –I to +I.
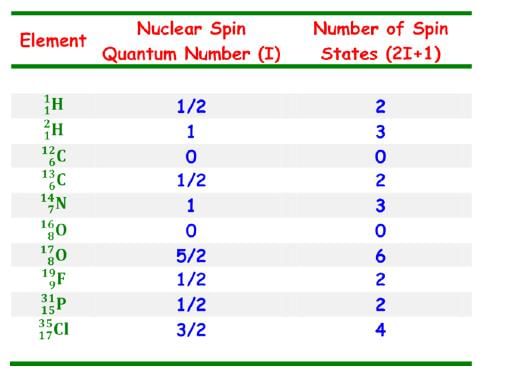
External Magnetic Field
When placed in an external field, spinning protons act like bar magnets.
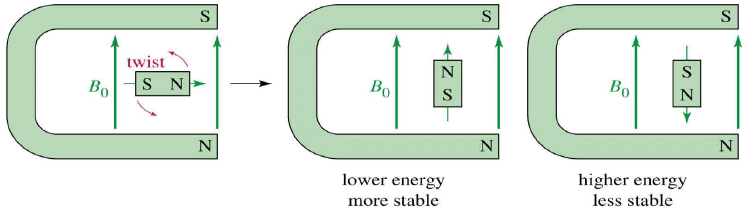
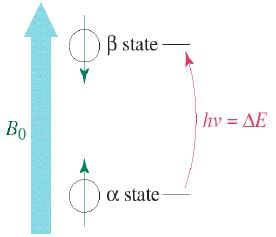
Two Energy States
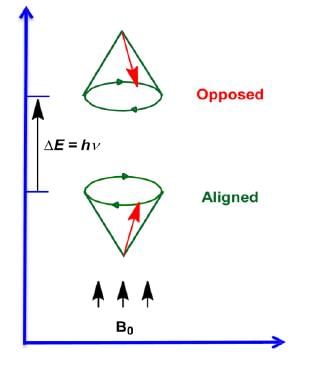
The magnetic fields of the spinning nuclei will align either with the external field, or against the field.
A photon with the right amount of energy can be absorbed and cause the spinning proton to flip.
Precessional Motion
- Since a proton is a spinning magnet, it moves in a characteristic fashion under influence of external magnetic field.
- This motion is called precessional motion, which is comparable to the motion of a spinning top.
ΔE and Magnet Strength
- Energy difference is proportional to the magnetic field strength.

- Gyromagnetic ratio (γ) is a constant for each nucleus (26,753 s–1gauss–1 for H).
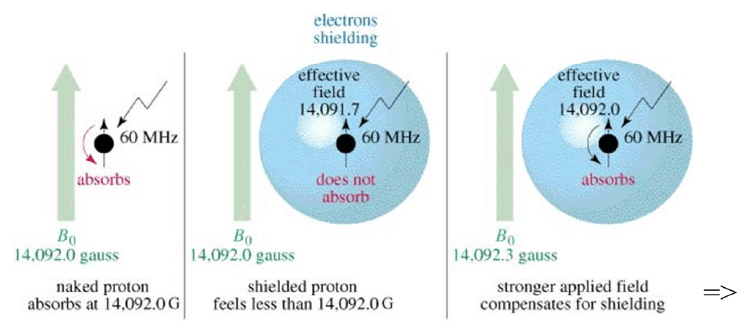
- In a 14,092 gauss field, a 60 MHz photon is required to flip a proton.
- Low energy, radio frequency.
Mechanism of Resonance
Under the effect of an applied magnetic field, nuclei start precessing along its axis with an angular frequency ω (Larmor Frequency).
- The nuclei being charged, generates an oscillating electric field having the same frequency ω.
- Now if radiowaves of the same frequency are supplied to this precessing nucleus, the two electric fields can couple and the energy can be absorbed by the nucleus. This process is called resonance.
- Resonance is accompanied by a change in spin of the nucleus.
Magnetic Shielding
• If all protons absorbed the same amount of energy in a given magnetic field, not much information can be obtained.
• But protons are surrounded by electrons that shield them from the external field.
• Circulating electrons create an induced magnetic field that opposes the external magnetic field.
Shielded Protons
Magnetic field strength must be increased for a shielded proton to flip at the same frequency.
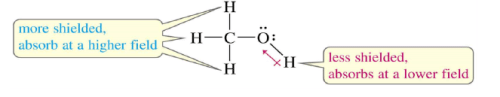
Protons in a Molecule
Depending on their chemical environment, protons in a molecule are shielded by different amounts.
The NMR Spectrometer
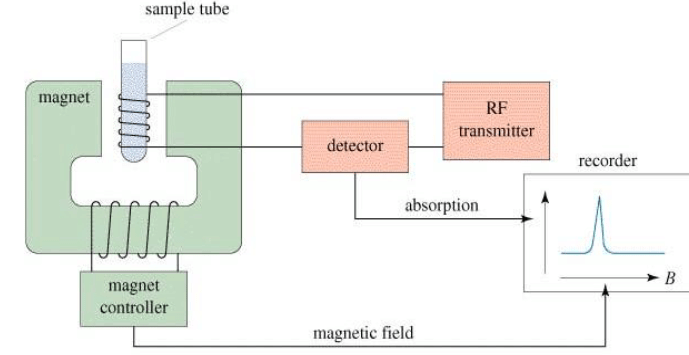
The NMR Graph
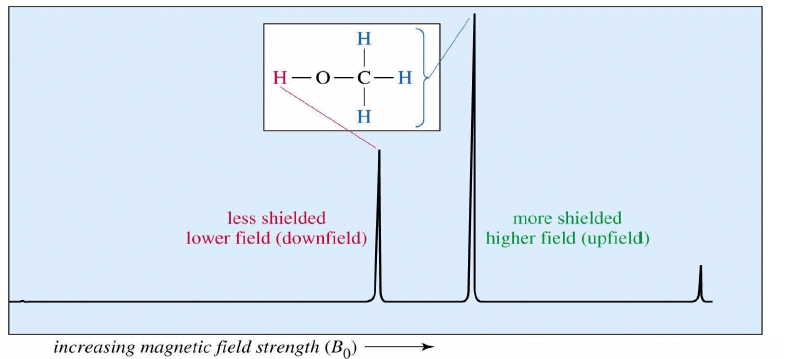
Tetramethylsilane (TMS)
- TMS is added to the sample.
- Since silicon is less electronegative than carbon, TMS protons are highly shielded. Signal defined as zero.
- Organic protons absorb downfield (to the left) of the TMS signal.
Criteria of Choosing Internal Standard:
- Should have equivalent nuclei (eg. In TMS all 12 H’s are equivalent).
- Should be chemically inert.
- Should have low boiling point, so that samples could be recovered.
- Should be soluble in most organic solvents.
- Should give one sharp signal in spectrum.
- TMS is commonly used for 1H, 13C NMR experiments.
- CFCl3 is used for 19F NMR experiments.
- Concentrated H3PO4 is used for 31P NMR experiments.
Delta Scale

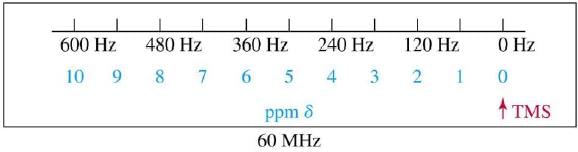

Location of signal
- More electronegative atoms deshield more and give larger shift values.
- Effect decreases with distance.
- Additional electronegative atoms cause increase in chemical shift.
Typical Values
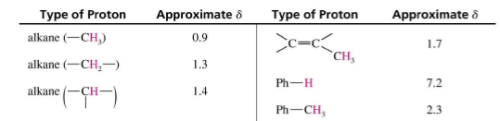
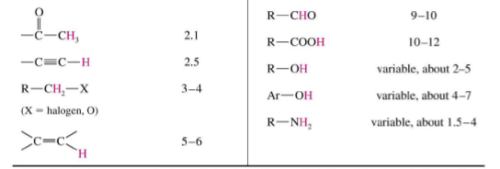

Factors Influencing Chemical Shifts
1. Electronegativity Effect:
- Electronegative element attached to a nucleus, increases its chemical shift value.
- Electron withdrawing groups attached to a carbon, withdraws valence electron density around the protons attached to that carbon.
2. Mesomeric Effect:
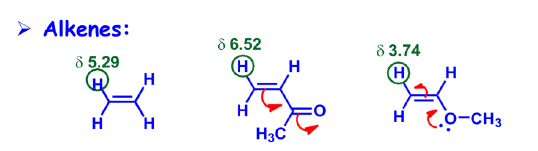
3. Hybridization Effect:
Order of electronegativity: sp > sp2 > sp3 (Consider % of s-character).
- Protons attached to sp3 carbons generally come within 0-2 ppm range.
- Normal vinylic protons resonate within 5.5-7.0 ppm.
- Aldehyde protons resonate within 9.0-10.0 ppm.
- sp2 carbons(33% s) are more electronegative than sp3 carbons (25% s) !!
- But only electronegativity can’t explain the reason for having so higher δ values than aliphatic protons. Another effect, called magnetic anisotropy need to be considered
4. Magnetic Anisotropy
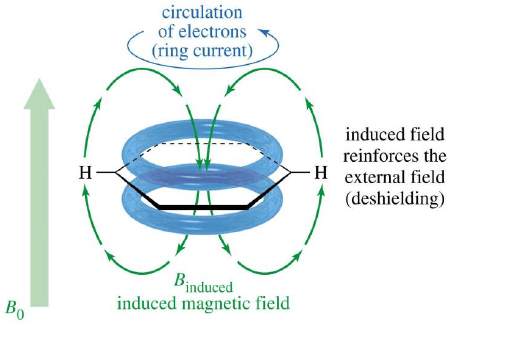
Aromatic Protons, δ7-δ8
When placed under a magnetic field, benzene π electrons are induced to circulate around the ring. This circulation is called ring current. The circulating electrons generate a magnetic field that creates a diamagnetic shielding zone inside the ring and paramagnetic deshielding zone in periphery of the ring.
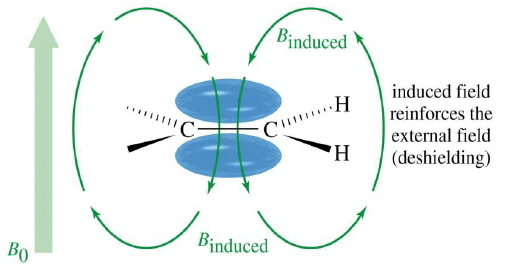
Vinyl Protons, δ5-δ6
Alkynic protons
Although sp carbons are more electronegative than sp2 carbons, alkynic protons resonate in much upfield (lower δ value) range than olefinic protons. In alkynes, the induced magnetic field generated due to circulating π electrons, shields alkynic protons.
So, the alkynic protons experience much less magnetic field strength than olefinic protons, as a result they appear in spectrum with a low δ value.

Aldehyde Proton, δ 9-δ 10
The aldehyde proton directly falls under paramagnetic deshielding zone due to anisotropy of C=O bond π electrons.
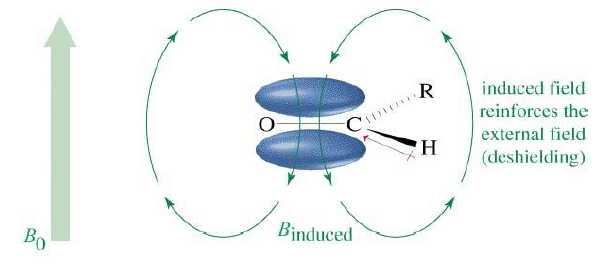
Annular protons
Annular protons reside in diamagnetic shielding zone, whereas outer protons fall in paramagnetic shielding zone.
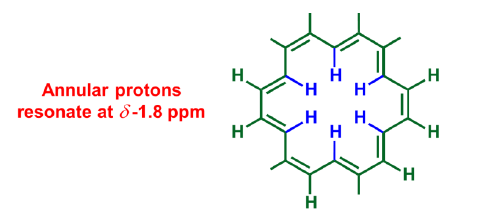
Others-

5. Hydrogen Bonding:
- Protons that can exhibit H-bonding, exhibit variable absorption over a wide range.
- H-bonding involves electron-cloud transfer from H-atom to nearest electronegative atom. So, H-bonded H’s experience a deshielding effect.
- The more H-bonding takes place, the more deshielded the proton becomes.
- Intramolecular H-bonding remains unchanged after dilution. So, chemical shift values also do not change after dilution.
- Carboxylic acids are stable in their dimeric form, which even persist in very dilute solution.
6. Effect of Solvents:
- In polar solvents, there will be much stronger dipolar interaction between solute & solvent specially in polar compounds.
- Interaction between a polar solvent and a polar solute is more than that of TMS, leading to a shift in δ value with respect to TMS.
- If the solvent has a strong magnetic anisotropy, solute-solvent interaction will also lead to significant shift in δ value.
The N + 1 Rule
If a signal is split by N equivalent protons, it is split into N + 1 peaks.
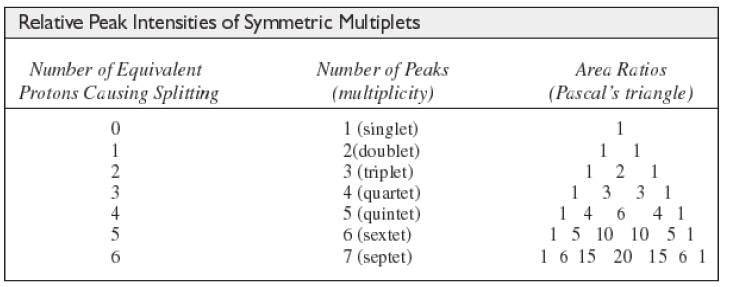
Intensity of Signals
- The area under each peak is proportional to the number of protons.
- Shown by integral trace.
Spin-Spin Splitting
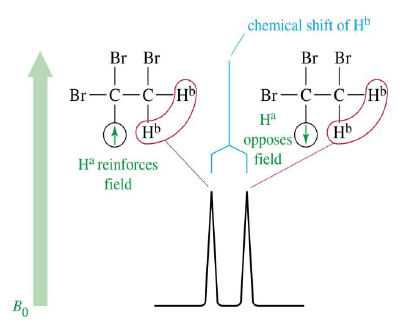
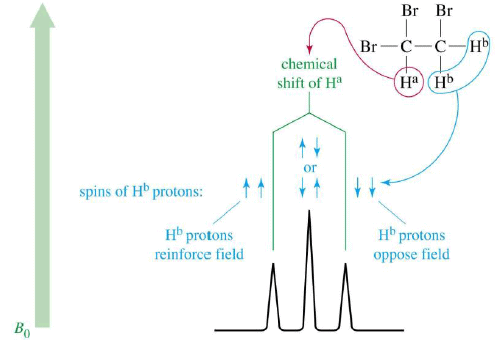
- Nonequivalent protons on adjacent carbons have magnetic fields that may align with or oppose the external field.
- This magnetic coupling causes the proton to absorb slightly downfield when the external field is reinforced and slightly upfield when the external field is opposed.
- All possibilities exist, so signal is split.
1,1,2-Tribromoethane: Nonequivalent protons on adjacent carbons.
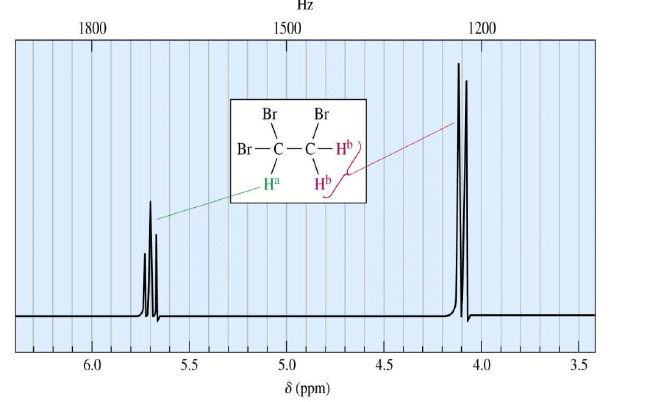
Doublet: Adjacent Proton
Triplet: Adjacent Protons
|
35 videos|92 docs|46 tests
|
FAQs on Nuclear Magnetic Resonance (NMR) Spectroscopy - Organic Chemistry
| 1. What is Nuclear Magnetic Resonance (NMR) spectroscopy? |  |
| 2. How does NMR spectroscopy work? |  |
| 3. What are the applications of NMR spectroscopy? |  |
| 4. What are the advantages of NMR spectroscopy? |  |
| 5. What are the limitations of NMR spectroscopy? |  |

|
Explore Courses for Chemistry exam
|

|