Pericyclic Reactions in Details (Part - 1) | Organic Chemistry PDF Download
1. Electrocyclic Ring Closure/Ring Opening
- Cyclization of an acyclic conjugated polyene system
- The terminal carbons interact to form a sigma bond
- Cyclic transition state involving either 4n electrons or 4n+2 electrons
Factors to consider
- The number of electrons involved has a profound influence on reactivity
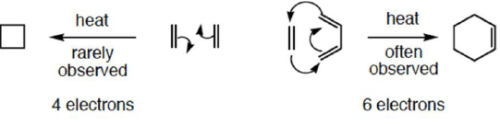
- Pericyclic reactions are stereospecific
- Reactions behave differently depending on the conditions used (i.e. thermal versus photochemical conditions)
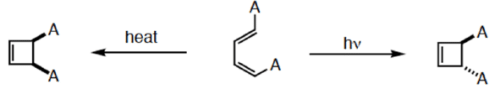
How do you rationalize?
Three theories are commonly used to explain pericyclic reactions.
1. Woodward-Hoffmann: Conservation of Orbital Symmetry
- First theory to explain and predict the outcome of many reactions
- Correlation diagrams
2. Dewar-Zimmerman: Aromatic Transition States
- The easiest to apply for all reaction types, but it is not as easy to understand why is it valid
- Aromatic or antiaromatic transition states
3. Fukui: Frontier Molecular Orbital Interactions
- Much easier to use than the original orbital symmetry arguments
- HOMO/LUMO interactions.
Need to understand Molecular Orbital Theory
Constructing MO diagram of polyene systems
1. Although there may be a change in the hybridization of carbon atoms during the course of a pericyclic reaction, the MO levels of the sigma framework are relatively unaffected. The p MOs can be constructed independently of C-C and C-H sigma bonds present.
2. For a conjugated polyene system containing n (n =even) p electrons, there will be n/2 p bonding
molecular orbitals that are filled MOs and n/2 antibonding MOs that are empty in the ground state electronic configuration of the molecule.
3. The lowest energy MO has zero nodes, the next higher one has one node and the second higher has two nodes and so on. The nth MO will have (n-1) nodes.
4. The nodal points are found at the most symmetric points in a MO. In other words, no MO can be symmetric as well as antisymmetric at the same time with respect to any existing molecular symmetry element. For example the π2 MO of butadiene has a node at the center of the bond connecting C2 and C3. It is incorrect to assign this node to the center of the bond connecting C1 and C2
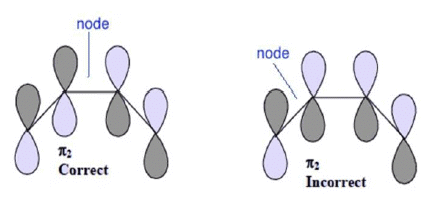
Important points to note:
- One could use ψ1, ψ2, ψ3… instead of π1, π2, π3…
- One could use signs + and – in place of using shading to denote change is phase of the orbital. Shading is preferred as it avoids confusion with charges.
- The line at the centre denotes atomic orbital level or non-bonding orbital level
- MOs can be constructed for odd no. of p-orbitals as well using same principles.
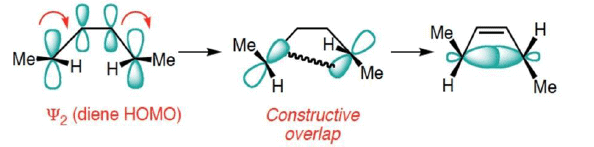
The Signs on the Outermost Lobes Must Match to Interact
- The lobes of like sign can be either on the same side or on opposite sides of the molecule.
- For a bond to form, the outermost lobes must rotate so that favorable bonding interaction is achieved
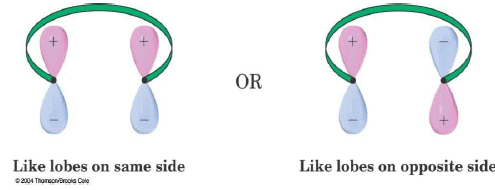
Some terminology
Ring closure can occur in two distinct ways. This has consequences with regard to:
- The orbital lobes that interact
- The disposition of substituents on the termini
Disrotatory Closure: The termini rotate in the opposite direction
Conrotatory Closure: The termini rotate in the same direction
1. Disrotatory Orbital Rotation
- If two lobes of like sign are on the same side of the molecule, the two orbitals must rotate in opposite directions—one clockwise, and one counterclockwise
- Woodward called this a disrotatory (dis-roh-tate’-or-ee) opening or closure
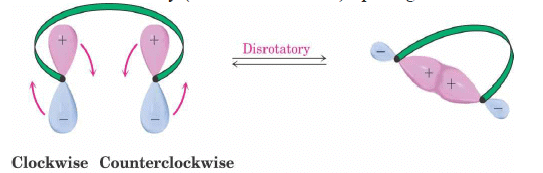
2. Conrotatory Orbital Rotation
- If lobes of like sign are on opposite sides of the molecule: both orbitals must rotate in the same direction, clockwise or counterclockwise
- Woodward called this motion conrotatory (con-roh-tate’-or-ee)
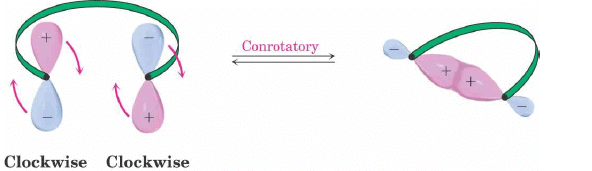
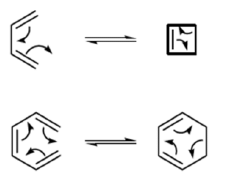
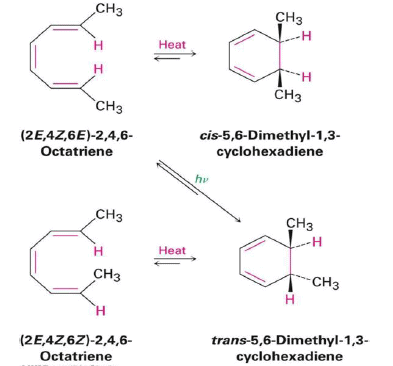
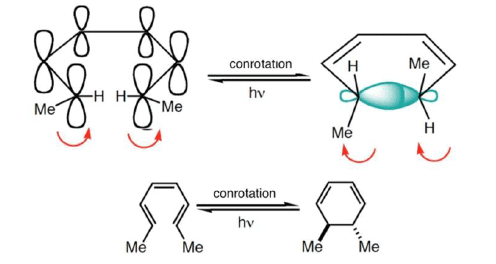
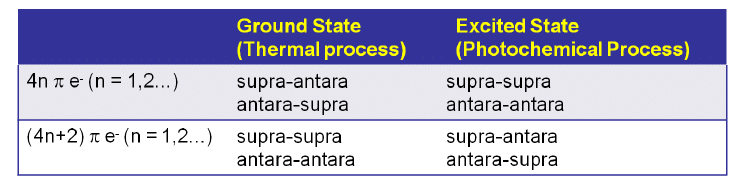
4 π-electron system and stereochemical issues
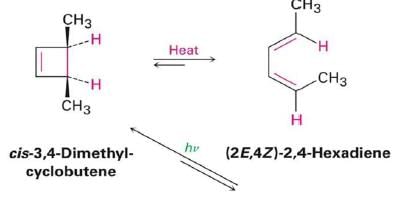
- Under thermal conditions, 4π-electron systems undergo conrotatory closure.
- The microscopic reverse reactions (i.e. ring opening) also occur with the same conrotatory sense.
- Changing the "reagent" from heat to light reverses this reactivity pattern.
- Under photochemical conditions, 4π-electron systems undergo disrotatory closure.
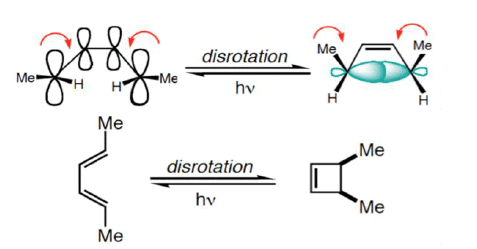
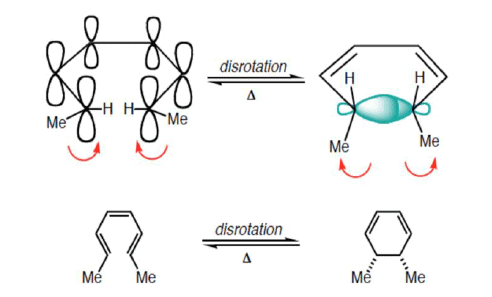
6π-eletcron system stereochemical issues
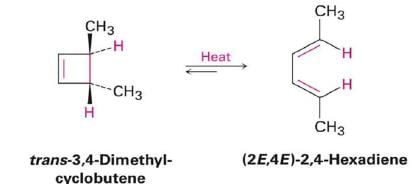
- Under thermal conditions, 6π-eletcron system undergo disrotatory closure
- The microscopic reverse reactions (i.e. ring opening) also occur with the same disrotatory sense
- Changing the "reagent" from heat to light reverses this reactivity pattern.
- Under photochemical conditions, 6π-electron systems undergo conrotatory closure.
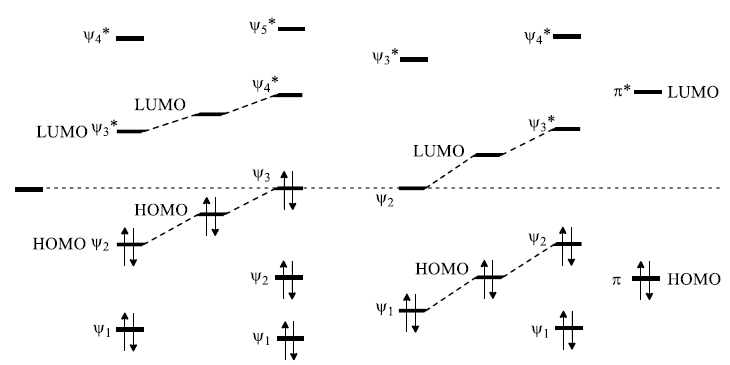
Frontier Molecular Orbital (FMO) Treatment of Electrocyclic reactions: Thermal Activation
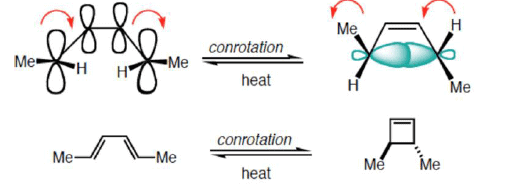
- Conrotatory Closure: (Allowed and observed)

- Disrotatory Closure: (Forbidden and not observed)
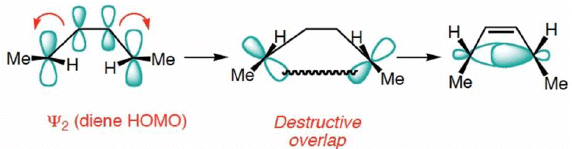
A similar analysis for the Hexatriene system, proves that under thermal conditions, disrotation is allowed and conrotation is forbidden.
FMO Treatment of Electrocyclic reactions: Photochemical Activation
- When light is used to initiate an electrocyclic reaction an electron is excited from Ψ2 to Ψ3.
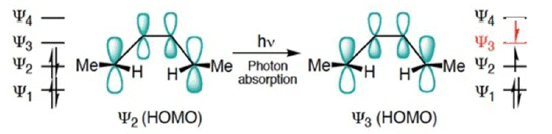
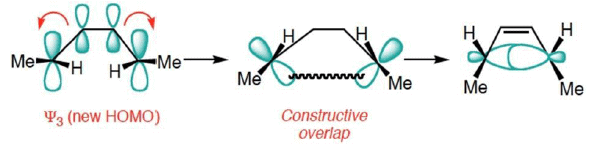
- Treating Ψ3 as the HOMO, now shows that disrotatory closure is allowed and conrotatory closure is forbidden.
- Disrotatory Closure: (Allowed and observed)
- Conrotatory Closure: (Forbidden and not observed)
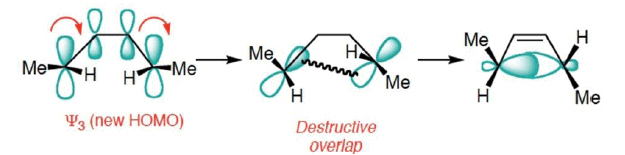
FMO Treatment of Electrocyclic reactions: Ring Opening Case
- We have so far proven which ring closures are allowed and which are forbidden. What about Ring openings?
- Do we now have to go back and examine all the ring openings?
o NO
- The principle of microscopic reversiblity says that if the reaction is allowed in one direction, it must be allowed in the other direction.
Electrocyclic Ring Opening to Diene
- Conjugated dienes and conjugated trienes react with opposite stereochemistry.
- Different symmetries of the diene and triene HOMOs.
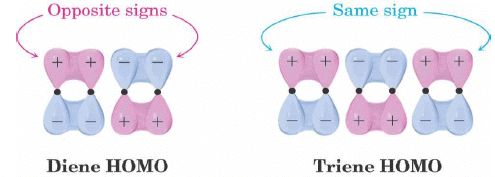
Selection Rules

2. CYCLOADDITION REACTIONS
- A cycloaddition reaction is the union of two smaller, independent π systems. Sigma bonds are created at the expense of π bonds.
- Cycloaddition reactions are referred to as [m + n] additions when a system of m conjugated atoms combines with a system of n conjugated atoms.
- Some examples are [2+2], [4+2], [4+4], [6+4], [6+6], [8+2]
- A cycloreversion is simply the reverse of a cycloaddition.
- [2+2] cycloaddition
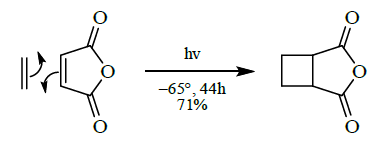
- [4+2] cycloaddition
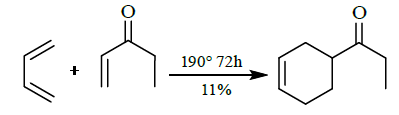
- [4+4] cycloaddition
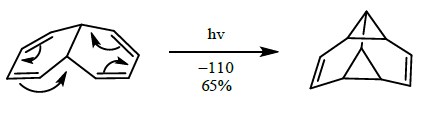
- [6+4] cycloaddition

- [6+6] cycloaddition

- [8+2] cycloaddition
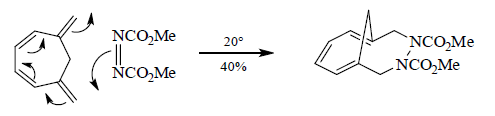 .
.
The Stereochemical issues
In a cycloaddition, a π system may be attacked in one of two distinct ways.
- If the π system is attacked from the same face, then, the reaction is suprafacial on that component.
- If the system is attacked from opposite faces, then, the reaction is antarafacial on that component.
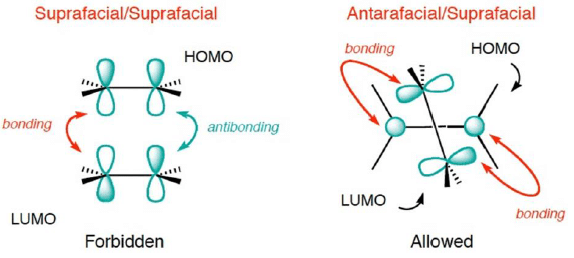
A. FMO Treatment of [2+2] Cycloaddition reaction
- Consider the reaction between two ground state ethylene molecules in which both of them undergo reaction in a suprafacial mode.
- HOMO of one ethylene interacts with LUMO of other ethylene.
Thermal activation:
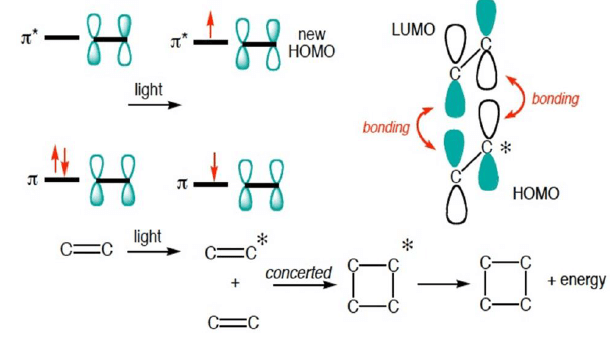
Photochemical Activation:
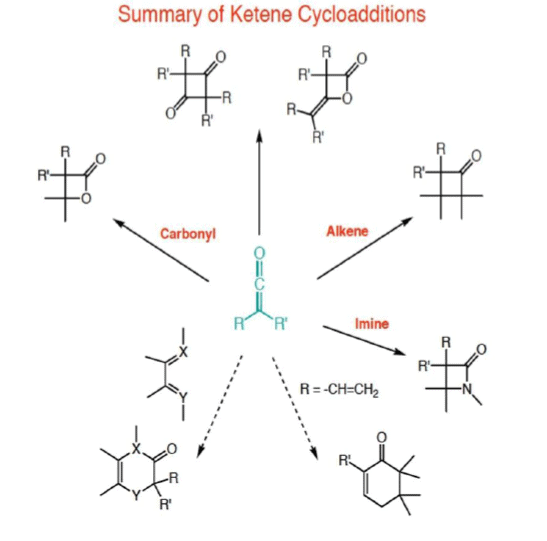
Ketene Cycloaddition Reactions
B. FMO Treatment of [4+2] Cycloaddition reaction


Some examples:
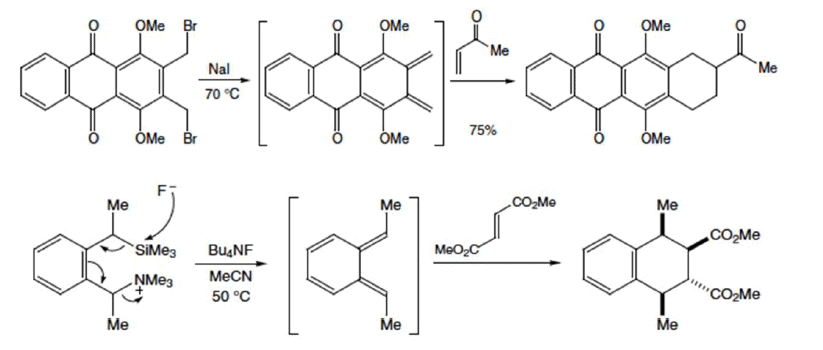
Selection Rules
 .
.
Components of Diels-Alder reaction:
1. Dienes
2. Dienophiles
3. Activations of Diels-Alder reaction by Lewis Acid
4. Regioselectivity of Diels-Alder reactions
5. Stereochemistry of Diels-Alder reactions
6. Retro Diels-Alder reactions
7. Intramolecular Diels-Alder reactions
8. 1,3-dipolar cycloaddition reactions
1. Dienes
- Diene must be able to adopt the s-cis geometry in order for cycloaddition to occur.
- Most impressive of all cyclic, dienes like cyclopentadiene are significantly more reactive than open-chain dienes.
- The s-trans conformation in a diene is lower in energy than the s-cis, with butadiene itself having only about 1% of its molecules at room temperature in the s-cis conformation.
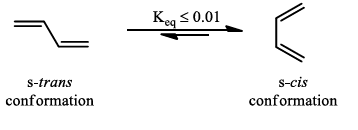
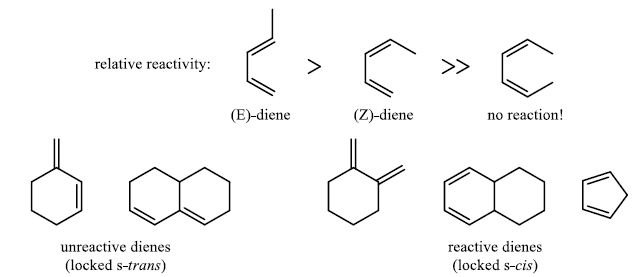
In electron-demand Diels-Alder reactions, dienes are activated by electron-donating substituents, such as alkyl, –NR2, and –OR. Electron-rich dienes accelerate the reaction with electron-deficient dienophiles, as illustrated by the relative reactivity trend shown below.
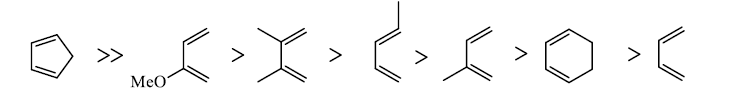
Danisliefsky's diene:
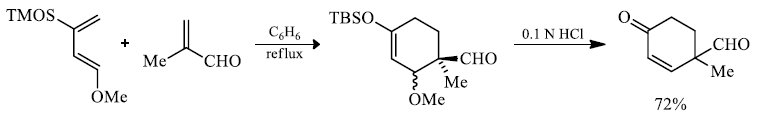
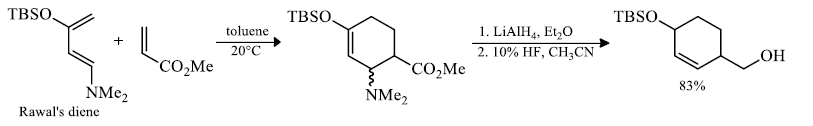
Rawal's dienes:
Brassard's diene:

Substitutent effect of diene and dienophile on rates of cycloadditions: A donor substituent on the diene raises the energy of its HOMO, and an electron-withdrawing substituent on the dienophile lowers the energy of its LUMO, bringing the two orbitals closer together in energy.
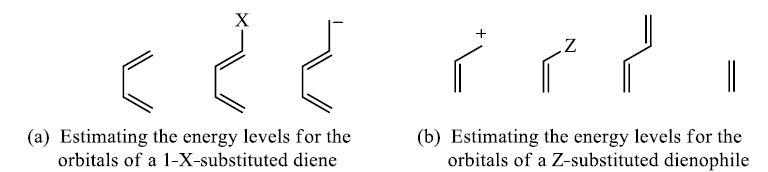
Lewis acid coordination to a carbonyl group will lower the LUMO energy even more and explain the large rate accelerations found for Lewis acid-catalysed Diels-Alder reactions.
2. Dienophiles:
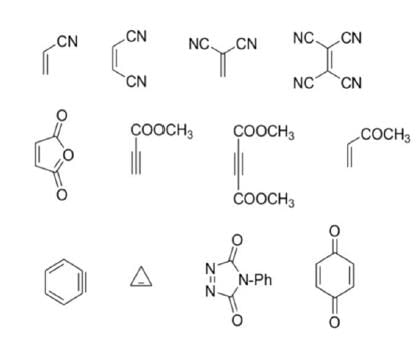
Dienophiles are activated by electron-withdrawing substitents. Alkyl groups, by means of inductive electron donation and steric effects, tend to reduce the rate of cycloaddition.
Relative dienophile reactivity
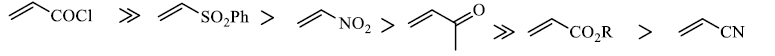
The reactivity of dienophiles may be increased by conjugation with additional electron-withdrawing groups. Doubly activated alkynes and 1,4-benzoquinones are particularly good participants in the Diels-Alder reaction.
Relative dienophile reactivity
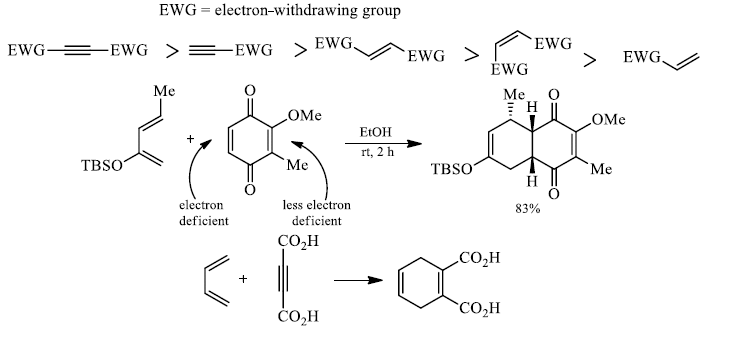
3. Activations of Diels-Alder reactions by Lewis Acid:
Many Diels-Alder reactions are accelerated by Lewis acid catalysts such as BF3.OEtZ2, AlCl3, Et2AlCl, SnCl4, TiCl4, and InCl3. These increase the rate of reaction by complexation with conjugated C = O and C = N groups in the dienophile.
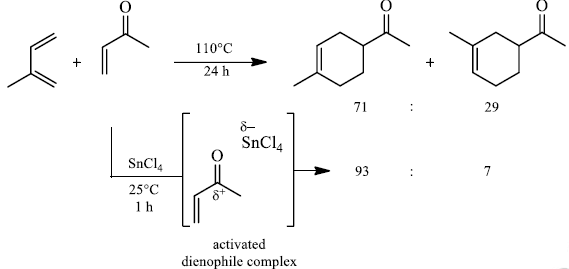
The reaction selectivity is often improved when using Lewis acid.

Lewis acid activation is particularly important for the catalysis of hetero-Diels- Alder reactions involving aldehydes.
Lanthanide complexes of Yb and Eu as well as samarium diiodide are mild catalysts for this reaction.
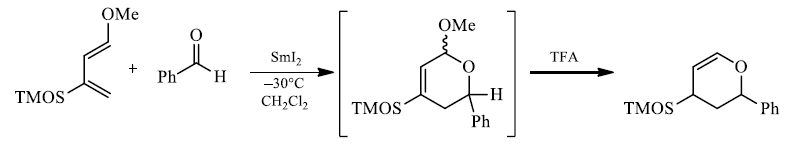
4. Regioselectivityof Diels-Alder reactions
The regioselectivity of Diels-Alder reactions ranges from moderate to very high. The cycloaddition reactions of monosubstituted dienes proceed with good selectivity. Generally, the more powerful the electronic effect of the diene substituent is, the more regioselective is the reaction.
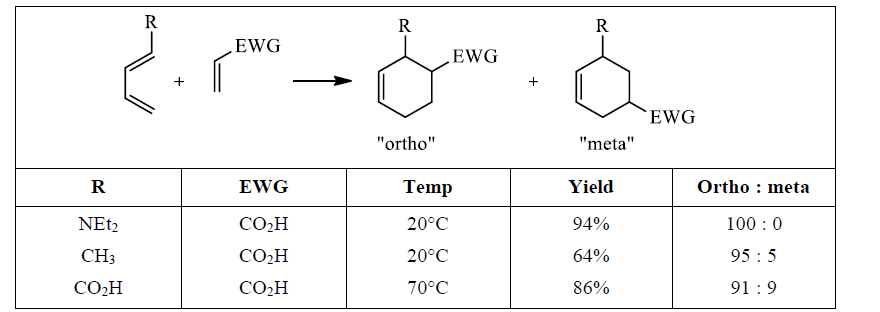
Table 1: 1-Substituted Butadienes React to Give Mainly the “ortho” Product.

A “simplistic” approach to predicting the regiochemical course of a Diels- Alder reaction is to consider the polarization of the diene and of the dienophile by examining the resonance forms, and then join the atoms with unlike charges to form a six-member ring, as exemplified below. However, this approach fails to account for some reactions that occur with good regiochernistry.
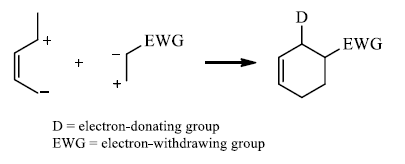
Some Examples
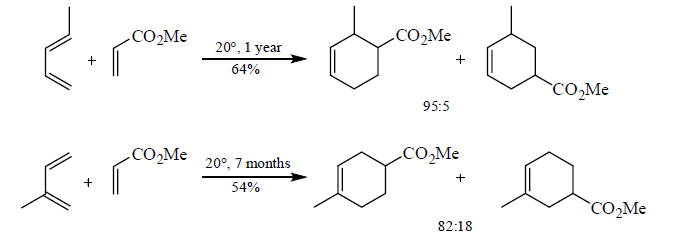
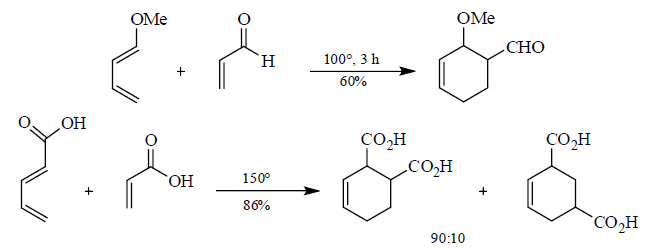
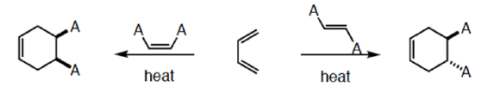
5. Stereochemistry of Diels-Alder reactions
An important aspect of the Diels-Alder reaction is its stereospecificity, wherein the relative
stereochernical relationships present in the starting materials are preserved throughout the course of the reaction.
Cis-principle.
The D-A reaction is concerted and suprafacial with respect to diene and dienophile.
Hence, the stereochemistries of both the diene and dienophile are retained in the adduct. Note that the initial suprafacial cycloadduct formed may in some cases be prone to isomerization. Suprafacial with respect to diene:
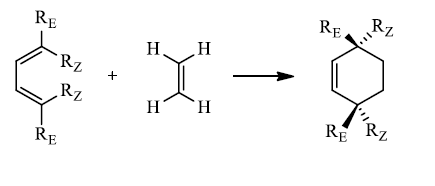
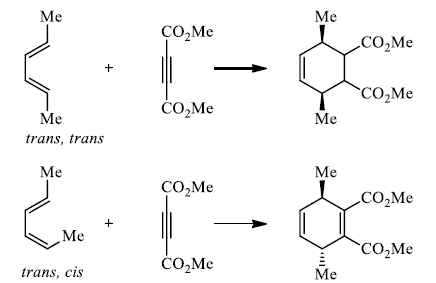
Suprafacial with respect to dienophile:
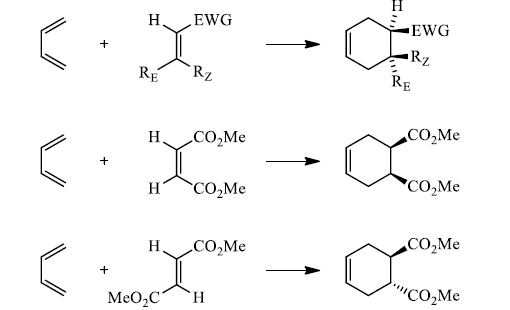
|
35 videos|92 docs|46 tests
|
FAQs on Pericyclic Reactions in Details (Part - 1) - Organic Chemistry
| 1. What are pericyclic reactions? |  |
| 2. How do pericyclic reactions differ from other types of reactions? |  |
| 3. What are the different types of pericyclic reactions? |  |
| 4. How can the conservation of orbital symmetry be used to predict pericyclic reactions? |  |
| 5. What are some applications of pericyclic reactions? |  |

|
Explore Courses for Chemistry exam
|

|