Lipids - Biomolecules | Organic Chemistry PDF Download
Lipid is a compound that is insoluble in water, but soluble in an organic solvent (e.g., ether, benzene,acetone, chloroform). ‘Lipid’ is synonymous with ‘fat’, but also includes phospholipids, sterols, etc.
Chemical structure: Glycerol + Fatty acids.
Function of Lipids
Lipids have four major functions which are physiologically important for human.
1. Serve as vitamins and hormones.
2. Structural components of biological membranes.
3. Synthesize bile acids that aid lipid solubilization.
4. Provide energy.
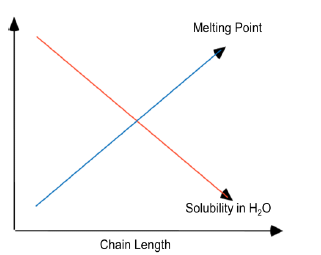
Melting Points and Solubility in Water of Fatty Acids
Classification of Lipids
1. Fatty acids: They are long chain linear (unbranched) hydrocarbon carboxylic acids
2. Triglycerides: They are fatty acid esters of glycerol
3. Phospholipids: They are lipids that contain one or more phosphate groups
4. Glycolipids: They have a carbohydrates component
5. Eicosanoids: They are a family of derivatives of Arachidonic acid
6. Steroids: They have a basic structure of a perhydrocyclo- pentanophenanthrene ring system
7. Lipoproteins: They are complexes of lipids and proteins that circulate in the blood.
Fatty Acids
They are long chain linear hydrocarbons carboxylic acids. They usually have an even number of C atoms (usually 12 to 20).The carbons are numbered starting from the carboxylic carbon. They are amphiphilic, they have a polar end and rest of the molecule is non-polar. Fatty acids may be saturated (no double bonds) or unsaturated (one or more double bonds). All naturally occurring double bonds have a cis-configuration. Two or more double bonds exist as non-conjugated double bonds. Longer chain and saturation increases melting point of FA. FAs are ionized at physiological pH.
Triglycerides
In Triacylglycerol (TG) all 3 –OH of glycerol are esterified by FAs. Monoacylglygerol and
diacylglycerol have, respectively, 1 and 2 FAs. Naturally occurring glycerol is L-glycerol. TG are the storage form of FA; most dietary fats are triglycerides. Physiologically, TG are digested in the small intestine by the enzyme pancreatic lipase. Monoacylglycerols are absorbed through the intestinal cells, re-converted to TG and assembled into lipoproteins.
Phospholipids
These are lipids that contain one or more phosphate groups. PL are the primary components of biomembranes. Other lipids in biomembranes are glycolipids and cholesterol. Surfactants are phopsholipids, mostly phosphatidylcholine.
Glycolipids are lipids that contain carbohydrates. Cerebrosides have a monosaccharide attached to the C1—OH of ceramide. Gangliosides have an oligosaccharide attached to the C1 —OH of ceramide. Cerebrosides and gangliosides are found in the brain and nervous tissue. In biomembranes, glycolipids are oriented asymmetrically with the sugar units always on the extracellular side of the membrane.
Eicosanoids
They are a family of powerful, hormone-like compounds. They are produced in the body from essential fatty acids. They have specific effects on target cells close to their site of formation. They are rapidly degraded, so they are not transported to distal sites within the body.
Examples: prostaglandins, prostacyclins, thromboxanes, leukotrienes, epoxyeicosatrienoic acids (EETs).
Roles in inflammation, fever, regulation of blood pressure, blood clotting, immune system modulation, control of reproductive processes & tissue growth, sleep/wake cycle regulation.
Steroids
General structure of a steroid has four rings.
Cholesterol are the derivatives of steroids. They are most abundant steroids in the body. Just add methyl CH3- groups, alkyl chain, and -OH to steroid nucleus.
Cholesterol
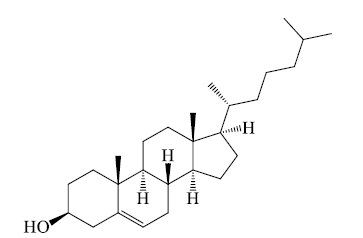
Cholesterol is an essential component of biomembranes and imparts stability to the fluid structure. Cholesterol is a steroid. All steroids have the same basic structure consisting of 4 hydrocarbon rings linked together. Cholesterol has a –OH group which provides the polarity and a hydrocarbon group at the other end which adds to its hydrophobic nature. In biomembranes, the –OH of cholesterol is aligned with the head group (phosphate) of phospholipids. Steroids are important metabolically (cholesterol), for digestion (bile salts), as hormones (human sex hormones).
Lipid Digestion/Absorption
Fats serve a structural function in cells, as sources of energy, and insulation.
The poor water solubility of lipids presents a problem for digestion as substrates are not easily accessible to digestive enzymes. Even if hydrolyzed, the products tend to aggregate to larger complexes that make poor contact with the cell surface and aren’t easily absorbed. To overcome these problems, changes in the physical state of lipids are connected to chemical changes during digestion and absorption.
Five different phases:
1. Hydrolysis of triglycerides (TG) to free fatty acids (FFA) and monoacylglycerols.
2. Solubilization of FFA and monoacylglycerols by detergents (bile acids) and transportation from the intestinal lumen toward the cell surface.
3. Uptake of FFA and monoacylglycerols into the cell and resynthesis to triglyceride
4. Packaging of TG’s into chylomicrons.
5. Exocytosis of chylomicrons into lymph.
Enzymes Involved in Digestion of Lipids
1. Lingual lipase: Provides a stable interface with aqueous environment of stomach
2. Pancreatic lipase: Major enzyme affecting triglyceride hydrolysis
3. Colipase: Protein anchoring lipase to the lipid
4. Lipid esterase: Secreted by pancreas, acts on cholestrol esters, activated by bile
5. Phospholipases: Cleave phospholipids, activated by trypsin.
|
35 videos|92 docs|46 tests
|
FAQs on Lipids - Biomolecules - Organic Chemistry
| 1. What are lipids? |  |
| 2. What are the main functions of lipids in the body? |  |
| 3. How are lipids digested and absorbed in the body? |  |
| 4. Are all lipids bad for health? |  |
| 5. How can lipids be measured in the body? |  |

|
Explore Courses for Chemistry exam
|

|

















