Introduction to Reaction Intermediates & Carbocations | Organic Chemistry PDF Download
| Table of contents |

|
| Reaction Intermediates |

|
| Carbocations |

|
| Classification of Carbocation |

|
| Generation of Carbocation |

|
| Fate of Carbocation |

|
| Stability of Carbocation |

|
| Reactions of Carbocation |

|
Reaction Intermediates
Reaction intermediates are generated by the breaking of covalent bond of the substrate. They are short-lived species and are highly reactive.
There are six types of reaction intermediates:
(1) Carbocation
(2) Carbanion
(3) Free radical
(4) Carbene
(5) Benzyne
(6) Nitrene

Carbocations
An organic species which has a carbon atom bearing six electrons in its outermost orbit and has a positive charge is called a carbocation.
- It has three bond pairs with empty p-orbital. Its hybridization is sp2.
- Shape of carbocation is trigonal planar.

[Note: Triphynylmethyl carbocation has propeller shape.]
- There are six electrons in the outermost orbit of carbocations carbon hence its octet is incomplete. All six electrons are paired.
- It is charged electrophile.
- It is diamagnetic in character.
- It is formed by heterolytic bond fission.
- It reacts with nucleophiles.
Classification of Carbocation
(i) Classical Carbocation: A classical carbocation is an ion containing a positively charged carbon atom which has six electrons that take part in three chemical bonds. We can name this carbon atom as a three-coordinate positive carbon.

To ensure maximum stability, the carbon atom should have eight valence electrons. But in the carbocation, there are only six electrons in the carbon atom having a positive charge. Therefore, it tends to share two more electrons from an electronegative species. This makes the carbon atom stable and neutralizes the positive charge. This is the reason for the high reactivity of classical carbocations. However, the energy of a classical carbocation is low compared to the energy of the corresponding nonclassical carbocation. But this difference in their energies is very small.
(ii) Non-Classical Carbocation: A nonclassical carbocation is an ion containing a positively charged carbon in a three-center two-electron center. This means, there are three atoms sharing two electrons in these carbocations. This type of electron sharing is named as delocalization of the electrons.

The most common example of a nonclassical carbocation is 2-norbornyl cation. It exists in a less symmetrical three-center two-electron structure. There is very little difference in the energy between classical and nonclassical carbocations. Therefore, it is very difficult to distinguish them experimentally.
Generation of Carbocation
Through direct ionisation of
bond where X leaves the molecule with bonding pair.
(i) From Alkyl halides: By SN1 reaction conditions, by Lewis acids

(ii) From alkene/alkynes:
By adding H+ (acids)like, H2SO4, H3PO4, TsOH, TfOH, BsOH etc. on alkene or alkyne.


Note: HNO3 is not used because it is oxidizing agent & oxidized alkene into aldehyde & ketones
(iii) From alcohol

(v) From Acyl halides:

Note: In some intramolecualr f.c. reactions (in Haworth synthesis etc.) both acylation & alkylation may give carbonation.

(vi) From Primary amine: 
[Note: Aromatic diazonium salts are more stable than aliphatic]
Fate of Carbocation
Carbocations are most often short-lived transient species and undergo three basic types of reactions.
(i) Combination with a nucleophile

(ii) Elimination of proton
Carbocation may loose a proton from the adjacent atom.

(iii) Addition to an unsaturated linkage

Stability of Carbocation
Stability of Carbocation can be gained by
- Ring expansion
- Ring contraction
- Rearrangement
- Aromaticity
- Resonance or mesomeric effect
- Inductive effect
- Hyperconjugation
Reactions of Carbocation
After formation of carbonation, we have to follow the following flow chart for the reaction.

(i) Stability of carbocation by ring expansion: It takes place when carbocation will formed adjacent to small ring



Another example-

(ii) Ring Contraction
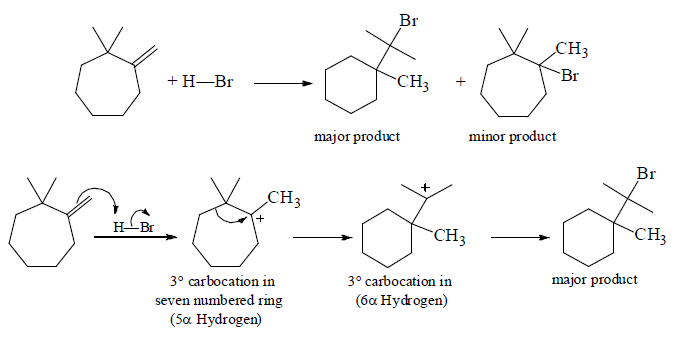
Another Example:

(iii) Rearrangement of carbocation in electrophilic addition reaction.
(a) By 1, 2-hydride shift

Mechanism of the 1, 2 hydride shift

(b) By 1, 2-methyl shift

(c) By 1, 2-phenyl shift

(iv) Stability of Carbocation by Aromaticity
(a) Cations in which positive charge is present on carbon of aromatic system is known as aromatic carbocation.
(b) Aromatic carbocations are so stable that even their solid states are known. For example, tropolium carbocations as tropolium bromide is a yellow solid. In fact tropolium carbocation is about 1011 times more stable than triphynylmethyl carbocation.
(c) Cations obeying Huckel (4n + 2) rule are stable because they are aromatic and there is complete delocalization of positive charge.

(v) Stability of carbocation by resonance:
(a) Allyl carbocation:
The stability of primary, secondary and tertiary allyl carbocations can be compared by
(a) Inductive effect (b) Hyperconjugation (c) Resonance

(b) Phynylmethyl carbocation
The stability of phenylmethyl carbocations can be explained by resonance.
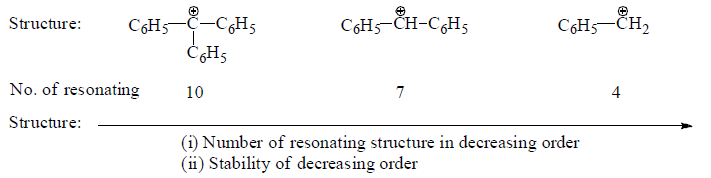
Phenylmethyl carbocations are more stable than allyl carbocations due to the number of resonating structures.
(c) Cyclopropylmethyl carbocation
(i) These carbocations are very stable carbocations. They are more stable than benzyl carbocations.
(ii) Stability of cyclopropyl methyl carbocations increases with every cyclopropyl group. Thus additions cyclopropyl group has a cumulative additive effect on the stability. Thus.

(iii) The special stability is a result of conjucation between the bent orbitals of the cycloproyl ring and the vecant-porbital of the cationic carbon. This type of bonding is called as banna bonding.


(e) Vinyl carbocation
When the positive charge is present on vinylic carbon then carbocation is known as vinyl carbocation;

This carbocation is the least stable because a positive charge is present on the electronegative carbon (sp-hybridized).
(vi) State of hybridization and stability
The positive charge is more stabilized on the less electronegative carbon atom. Hence, increasing s-character increases electronegativity, and its capability to stabilize positive charge decreases.

(decreasing stability with increasings character in its state of hybridization)
Alkyl carbocations
The stability of alkyl carbocations can be explained by:
(a) Inductive effect (b) Hyperconjugation
According to these inductive effects the stability order is as follows:

Bridge heads carbocations
Bridge heads cannot attain planar configuration. Therefore, a carbocation is never formed at the bridgehead.

Examples for illustration

(When + change is on the C of benzene ring then resonance effect don’t work)

Stability order: A < B < C < D

Stability order: A > C > D > B
(R effect is equal to at O & P but I effect is distance dependent)

Stability Order :D > B > A > C

Stability Order :D > B > A > C

Stability Order: C > A > B > E > F > D

Stability Order: D > E > A > C > B

Stability Order: D > C > A > F > E > B

Stability Order: F > E > C > A > B > D Wrong
Stability Order: F > E > C > D > B > A Right
|
35 videos|92 docs|46 tests
|
FAQs on Introduction to Reaction Intermediates & Carbocations - Organic Chemistry
| 1. What are reaction intermediates in chemistry? |  |
| 2. How are carbocations classified based on their structure? |  |
| 3. What are the common methods for generating carbocations? |  |
| 4. What are the possible fates of a carbocation once it is formed? |  |
| 5. Why is the stability of a carbocation important in chemical reactions? |  |

|
Explore Courses for Chemistry exam
|

|
 bond where X leaves the molecule with bonding pair.
bond where X leaves the molecule with bonding pair.


















