Metal Based Oxidizing Reagents (Part - 2) | Organic Chemistry PDF Download
Mn Based Oxidizing Reagents
Potassium Permanganate [KMnO4]
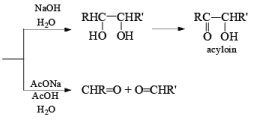
(a) Oxidation of Alkenes: The reaction of alkenes with alkaline potassium permanganate proceeds rapidly via formation of a cyclic manganese ester, which is hydrolyzed to the 1, 2-diol. To avoid over-oxidation to an acyloin (a-ketol), the pH of the reaction medium has to be monitored. Improved yields of diols are obtained when the oxidation is carried out in water in the presence of a phase transfer agent.
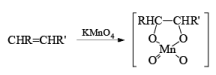
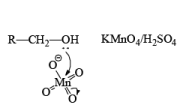
(b) Oxidation of Alcohol: The important condition which must be fulfilled is the presence of a-hydrogen for oxidation of alcohol.
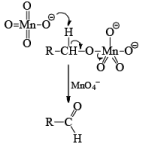
Mechanism: During the mechanism of oxidation of alcohol the O—H as well as C—H bond breaks. If the a hydrogen is not present then no oxidation will occur.
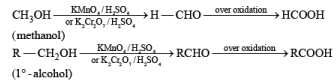
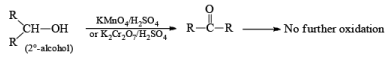
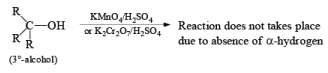
Potassium Permanganate and Co-oxidant: The less volatile and less expensive potassium permanganate may also be used for the cleavage of alkenes in the presence of periodate. However, if the cleavage of the resulting diol by periodate leads to an aldehyde fragment, it will be further oxidized by potassium permanganate to the corresponding carboxylic acids. Ketones are stable under potassium permanganate catalyzed oxidation
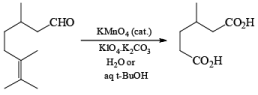
(c) Aromatic Hydrocarbons: When any aromatic hydrocarbon is used in which alkyl groups are present then it is possible to oxidize the chain of alkyl group into –COOH group but the oxidation always occurs at benzylic position and at least one benzylic hydrogen must be present for oxidation.
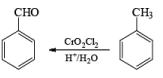
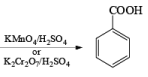
Manganese Dioxide (MnO2): MnCl2 + KMnO4 + H2O → MnO2 (ppt)
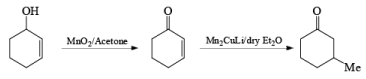
MnO2 is a highly chemoselective oxidant-allylic, benzylic, and propargylic alcohols are oxidized faster than saturated alcohols. The oxidation takes place under mild conditions in H2O, acetone, or CHCl3 If allylic or benzylic alcohol is not present it will very slowly oxidize the other alcohol.
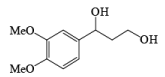
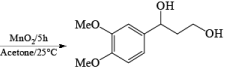
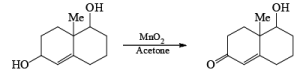
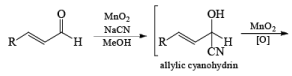
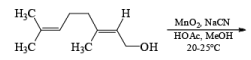
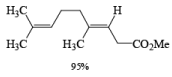
α, β-Unsaturated aldehydes are converted directly to carboxylate esters by MnO2 and NaCN in an alcohol solvent. Sodium cyanide catalyzes the oxidation by forming a cyanohydrin that is susceptible to MnO2 oxidation.
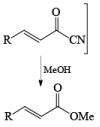
Methanolysis of the acyl cyanide intermediate in the example below gives the methyl ester in excellent yield.
The oxidation of allylic alcohols directly to methyl carboxylates has been reported using MnO2 and sodium cyanide in methanol.
Ag Based oxidizing agent:
Silver Carbonate on Celite (Fetizon’s Reagent)
Fetizon’s Reagent is chemoselective for the oxidation of 2°-alcohol in presence of primary alcohol.
The silver carbonate is the oxidizing reagent adsorbed on the celite resin. Highly hindered –OH groups are not oxidized. Oxidation of allylic a, b-diols gives high yields of five- or six-member ring lactone.
Rate of oxidation of –OH group
Benzylic ; Allylic > 2°-Alcohol >> 1°-Alcohol
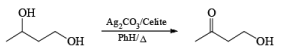
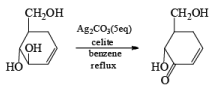
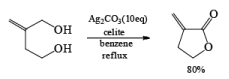
- In the case of 1, 4 diol 1, 5 diol or 1, 6 diol when fetizon’s reagent is used then it will form lactone just like PCC.

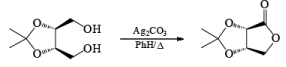
Silver carbonate has been used to oxidize lactols (hemiacetals) to lactone.
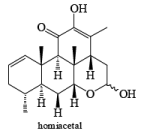
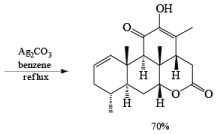
Tollens Reagent:
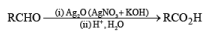
Osmium Tetroxide
The dihydroxylation of olefins with OsO4 (a very toxic and volatile reagent that must be handled with care) provides a reliable method for the preparation of cis-1,2-diols. Although osmium tetroxide is expensive, it is the reagent of choice for syn-dihydroxylation because yields of diols obtained are usually high.
The reaction can be depicted as a concerted syn-addition of the reagent to the double bond, forming the cyclic osmate ester, which upon hydrolysis or reduction (H2S, NaHSO3, or Na2SO3) produces the cis- 1,2-diol.
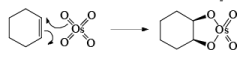
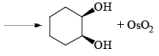
The reaction is stereospecific in that (E)-alkenes furnish the syn-1,2-diols, whereas (Z)-alkenes give the anti-1,2-diols.
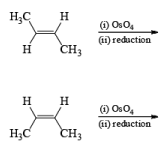
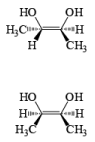
double bond, but appears to be less susceptible to steric hindrance than epoxidation with peroxy acids.
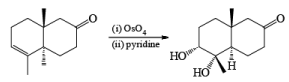
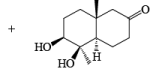
Osmium-tetroxide-catalyzed dihydroxylation of sterically hindered olefins proceeds more efficiently with trimethylamine N-oxide in the presence of pyridine. The base appears to catalyze not only formation of the osmate ester, but also its hydrolysis.
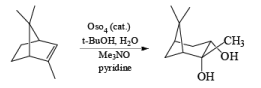
Under controlled conditions, the osmium-tetroxide-mediated dihydroxylation is also chemoselective, reacting preferentially with the more nucleophilic double bond in the presence of a triple bond.
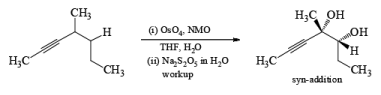
syn-Dihydroxylation on the more hindered side of cycloalkenes can be achieved with iodine and silver acetate in moist acetic acid (Woodward procedure) or more economically with iodine and potassium iodate in acetic acid
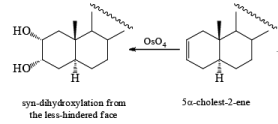
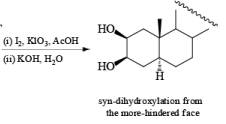
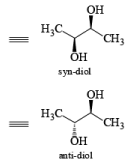
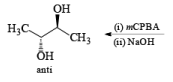
It should also be noted that epoxidation of olefins followed by ring opening with OH– provides 1,2 diols with different stereochemistry from that observed with osmium-tetroxide-mediated syn-dihydroxylation. Therefore, even when only one diastereomeric olefin is available, it is possible to prepare both diastereorneric 1, 2-diols, as shown below.
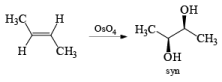
Osmium Tetroxide with Co-oxidant: A useful alternative to ozonolysis, especially when carried out on a large scale, is the oxidation of alkenes with a catalytic amount of OsO4 (1-5 mol %) in the presence of NaIO4 or KIO4 as co-oxidants (Lemieux- Johnson oxidation). The periodate has a dual function: cleavage of the 1,2-diol (glycol) formed by the oxidizing agent and re-oxidation of the reduced osmium dioxide to osmium tetroxide. Periodate oxidation of the diol does not proceed beyond the aldehyde stage. Moreover, triple bonds are not affected by OsO4 or by periodate.
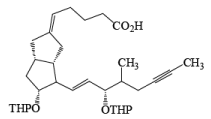
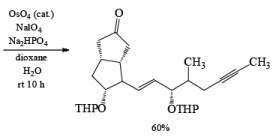
An isolated double bond will be cleaved selectively in competition with an electron-deficient conjugated double bond of an enone or of an a, b-unsaturated ester.
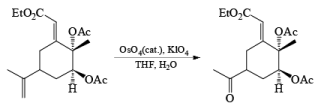
Formation of an OsO4· (pyridine), complex enhances the chemoselectivity of attack ;it thc electron-rich, casily accessible double bond in compounds with multiple double bonds.
Lead Tetraacetate: Pb(OAc) 4 may be used either in toluene or in THF for oxidation of glycols that are only slightly soluble in aqueous media. It accomplishes the same types of reactions effected by NaIO4 with H2O-soluble compounds. Being a more powerful oxidizing agent, Pb(OAc)4 will also cleave 1,2-diketones, a-hydroxy ketones, and a-hydroxy acids.
Oxymercuration Demercuration
Acid catalyzed hydration of alkenes is not well suited for laboratory preparation of alcohols. Since the reaction proceeds via carbocation intermediates, mixtures of alcohols may be formed. However, oxymercurationdernercuration of alkenes provides a simple tool for regioselective hydration of alkenes whereby rearrangements are seldom observed.
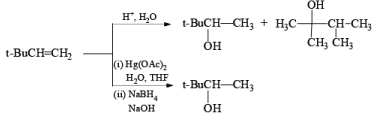
Treatment of an alkene with mercuric acetate in aqueous THF results in the electrophilic addition of mercuric ion to the double bond to form an intermediate mercurium ion. Nucleophilic attack by H2O at the more substituted carbon yields a stable organomercury compound, which upon addition of NaBH4 undergoes reduction. Replacement of the carbon-mercury bond by a carbon-hydrogen bond during the reduction step proceeds via a radical process. The overall reaction represents Markovnikov hyclration of a double bond, which contrasts with the hydroborationoxidation process.
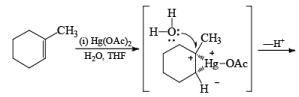
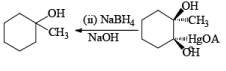
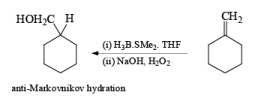
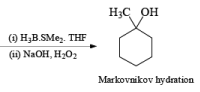
Solvomercuration-Demercuration
Mercuration of 1-alkenes in the presence of nucleophilic solvents such as alcohols, amines, and nitriles or in the presence of sodium azide provides convenient access to the corresponding ethers, amines, amides, and azides.
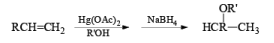
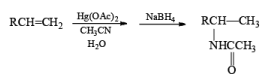
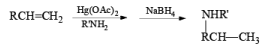
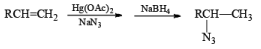
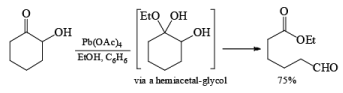
|
35 videos|92 docs|46 tests
|
FAQs on Metal Based Oxidizing Reagents (Part - 2) - Organic Chemistry
| 1. What are some common metal based oxidizing reagents? |  |
| 2. How do metal based oxidizing reagents work? |  |
| 3. What are some applications of metal based oxidizing reagents? |  |
| 4. Are metal based oxidizing reagents corrosive or hazardous? |  |
| 5. Can metal based oxidizing reagents be used in water treatment? |  |

|
Explore Courses for Chemistry exam
|

|


















