Introduction to Solutions, Ideal & Non Ideal Solutions | Physical Chemistry PDF Download
| Table of contents |

|
| Solutions |

|
| Types of Solutions |

|
| Vapour Pressure of a Solution |

|
| Raoult’s Law |

|
| Composition of the Vapour |

|
| Ideal solutions |

|
| Non-ideal Solutions |

|
Solutions
In chemistry, a solution is a special type of homogeneous mixture composed of two or more substances.
- The term aqueous solution is when one of the solvents is water. In such a mixture, a solute is a substance dissolved in another substance, known as a solvent.
- The mixing process of a solution happens at a scale where the effects of chemical polarity are involved, resulting in interactions that are specific to solvation.
- The solution assumes the phase of the solvent when the solvent is the larger fraction of the mixture, as is commonly the case.
- The concentration of a solute in a solution is the mass of that solute expressed as a percentage of the mass of the whole solution.
Characteristics of Solutions
- A solution is a homogeneous mixture of two or more substances.
- The particles of solute in a solution cannot be seen by the naked eye.
- A solution does not allow beams of light to scatter.
- A solution is stable.
- The solute from a solution cannot be separated by filtration (or mechanically).
- It is composed of only one phase.
Types of Solutions
Homogeneous means that the components of the mixture form a single phase. Heterogeneous means that the components of the mixture are of different phase.
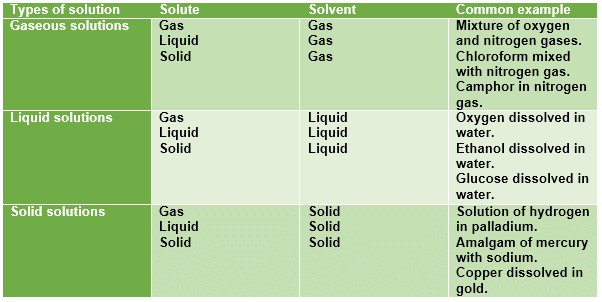
- The properties of the mixture (such as concentration, temperature, and density) can be uniformly distributed through the volume but only in absence of diffusion phenomena or after their completion.
- Usually, the substance present in the greatest amount is considered the solvent. Solvents can be gases, liquids, or solids.
- One or more components present in the solution other than the solvent are called solutes. The solution has the same physical state as the solvent.
Gaseous Solutions
- If the solvent is a gas, only gases are dissolved under a given set of conditions.
- An example of a gaseous solution is air (oxygen and other gases dissolved in nitrogen).
- Since interactions between molecules play almost no role, dilute gases form rather trivial solutions.
- In part of the literature, they are not even classified as solutions but addressed as mixtures.
Liquid Solutions
If the solvent is a liquid, then almost all gases, liquids, and solids can be dissolved. Here are some examples:
1) Gas in liquid:
- Oxygen in water
- Carbon dioxide in water – a less simple example, because the solution is accompanied by a chemical reaction (formation of ions).
- Note also that the visible bubbles in carbonated water are not the dissolved gas, but only an effervescence of carbon dioxide that has come out of solution; the dissolved gas itself is not visible since it is dissolved on a molecular level.
2) Liquid in liquid:
- The mixing of two or more substances of the same chemistry but different concentrations to form a constant. (Homogenization of solutions)
- Alcoholic beverages are basically solutions of ethanol in water.
3) Solid in liquid:
- Sucrose (table sugar) in water
- Sodium chloride (NaCl) (table salt) or any other salt in water, which forms an electrolyte: When dissolving, the salt dissociates into ions.
- Solutions in water are especially common and are called aqueous solutions.
- Non-aqueous solutions are when the liquid solvent involved is not water.
- Counterexamples are provided by liquid mixtures that are not homogeneous: colloids, suspensions, emulsions are not considered solutions.
- Body fluids are examples of complex liquid solutions, containing many solutes. Many of these are electrolytes since they contain solute ions, such as potassium. Furthermore, they contain solute molecules like sugar and urea.
- Oxygen and carbon dioxide are also essential components of blood chemistry, where significant changes in their concentrations may be a sign of severe illness or injury.
Solid Solutions
If the solvent is a solid, then gases, liquids, and solids can be dissolved.
1) Gas in solids:
- Hydrogen dissolves rather well in metals, especially in palladium; this is studied as a means of hydrogen storage.
2) Liquid in solid:
- Mercury in gold, forming an amalgam· Water in solid salt or sugar, forming moist solids · Hexane in paraffin wax
3) Solid in solid:
- Steel, basically a solution of carbon atoms in a crystalline matrix o f iron atoms
- Alloys like bronze and many others
- Polymers containing plasticizers
Vapour Pressure of a Solution
The pressure exerted by vapours over the liquid surface at this equilibrium is called vapour pressure of the liquid.
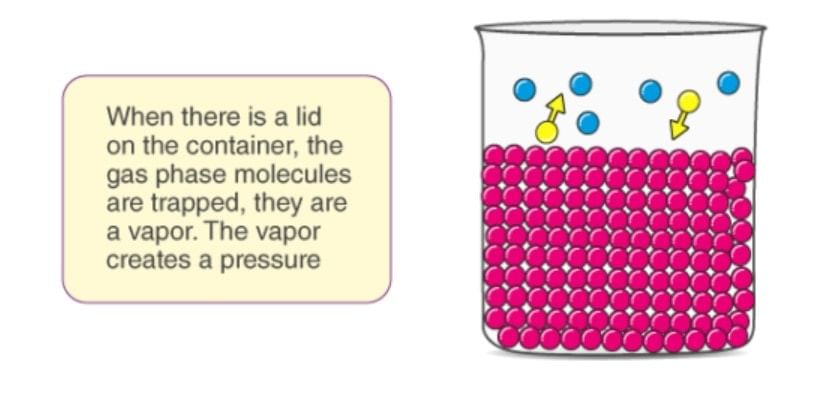
- Consider a pure liquid in a beaker is covered with a jar. Some molecules of the liquid on the surface evaporate and fill the vacant space available to them.
- The molecules in the vapour phase move randomly in the vacant space, during this motion, they strike the surface of the liquid and get condensed. This process of evaporation and condensation go on and an equilibrium is established between evaporation and condensation.
- The pressure exerted by vapours over the liquid surface at this equilibrium is called vapour pressure of the liquid.
- If the solution is nonvolatile solid or liquid the vapour pressure of solution is equal to partial vapour pressure of solvent in the solution and if the solute and if the solute is volatile solid or liquid, then a vapour pressure will be equal to the sum of partial vapour pressure of solute and that of solvent.
Raoult’s Law
This law is applied for a solution of liquid in liquids and can be stated as follows. “The partial vapour pressure of any component in the solution is directly proportional to its mole fraction”.
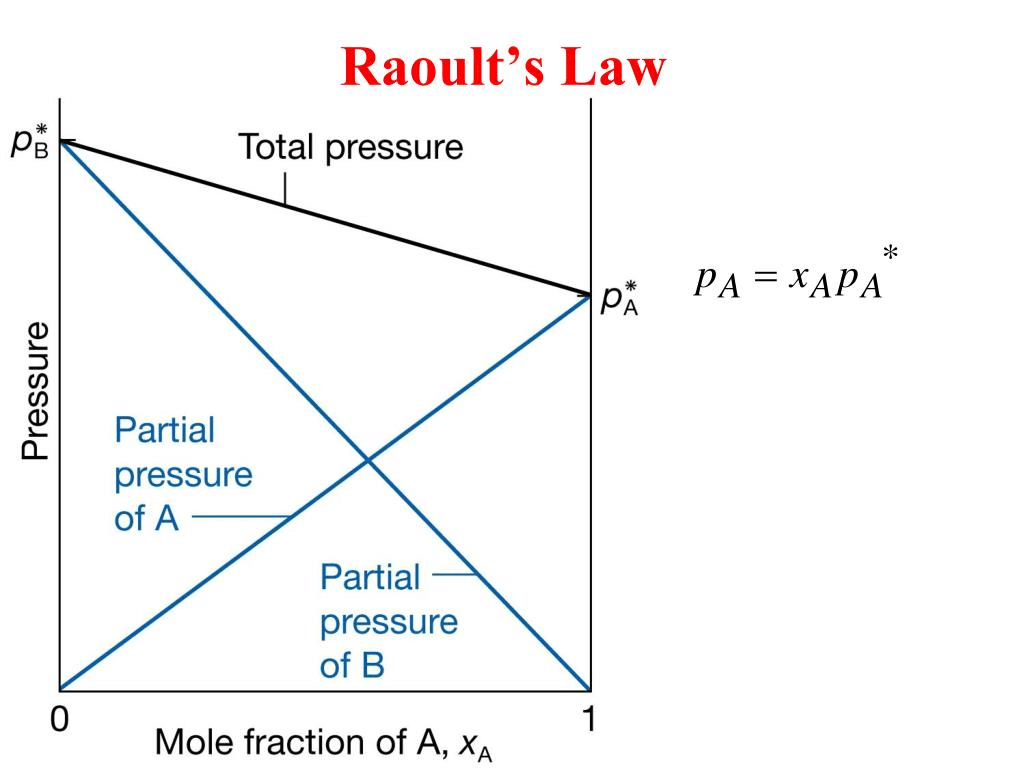
PA ∝ XA where PA = Partial Vapour pressure of XA
PA = KXA XA = Mole fraction of A in solution
For pure liquids XA = 1
Then K = P°A where P°A is the vapour pressure of component A is a pure state
Hence, PA = XAP°A, similarly for component B, PB = XBP°B
Raoult’s law in combination with Dalton’s law of partial pressure
Assuming that vapours of a liquid is behaving like an ideal gas, then according to Dalton’s law of partial pressure the total pressure PT is given by
PT = PA + PB
= XAP0A + XBP0B
= XAP0A + (1-XA) P0B = Vapour pressure in terms of Mole fraction of Solvent.
= P0B + (P0A - P0B)XA
Example: The vapour pressure of ethanol and methanol are 44.5 mm and 88.7 mm Hg respectively. An ideal solution is formed at the same temperature by mixing 60 g of ethanol with 40 g of methanol. Calculate total vapour pressure of the solution.
Solution: Number of moles of ethanol = 60/40 = 1.5
Number of moles of methanol = 40/32 = 1.25
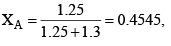 XB 1-0.4545 = 0.545
XB 1-0.4545 = 0.545
Let A = CH3OH, B = C2H5OH
Total pressure of the solution
PT = XAP°A + XBP°A = 0.4545 × 88.7 + 0.545 × 44.5 = 40.31 + 24.27 = 64.58 mm Hg
Composition of the Vapour
The composition of the liquid mixture and vapour that are in mutual equilibrium are not necessarily the same, the common sense suggest that the vapour pressure should be richer in the more volatile component. This expectation can be confirmed as follows:
Let the mole fraction of A and B in vapour phase be YA and YB then from Dalton’s law,
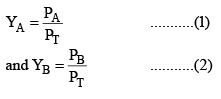
Provided the mixture of vapours behaves as an ideal gas
Rewriting equation (1)
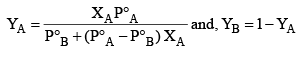
Example: What is the composition of the vapour which is in equilibrium at 30°C with a benzene toluene solution with a mole fraction of benzene of 0.400?
(P°B = 119 torr and P°T = 37.0 torr)
Solution: Total pressure of the solution is given by
PT = XBP°B + XTP°T
= 0.4 × 119 + 0.6 × 37 = 47.6 + 22.2
= 69.8 torr
Applying Dalton’s law for mole fraction is vapour phase.
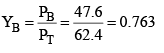
YT = 1 – 0.763 = 0.237
Condensation of Vapour of Solution
When the vapours of solution (containing liquids A and B is) condensed, the composition of liquids A and B in the condensate remains same. Vapour over condensate can again recondense and the composition of A and B in condensate (2) remains same as it was in vapour phase over condensate (1).
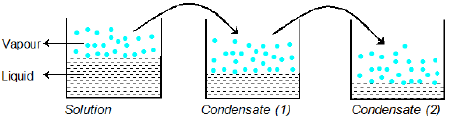
Example: Liquid A and B form an ideal solution. The vapour pressure of A and B at 100°C are 300 and 100 mm Hg respectively. Suppose that vapour above solution is composed of 1 mole of A and 1 mole of B is collected and condensed. This condensate is then heated at 100°C and vapour are again condensed to form a liquid L. What is the mole fraction of A in the vapours of L?
Solution: Vapour pressure due to vapours above solution
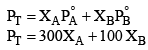
It is given that in vapour phase each of A and B are one mole each hence each of them have mole fraction 0.5 in vapour phase.
After condensation of vapours.
In condensate (1)
X'A 0.5, X'B 0.5
P'T 0.5 × 300 0.5 × 100 150 + 50 = 200 mm
Mole fraction A and B in vapour phase of condensate
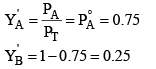
When the vapours of the condensate (1) will again be vaporized in condensate (2) liquid L.
X"A = 0.75, X"B = 0.25, where X"A and X"B are mole fraction of A and B in liquid L.
P"T = 300 × 0.75 + 100 × 0.25 = 225 + 25 = 250 mm and mole fraction of A in vapour phase of the condensate (2) is given by
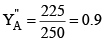
Ideal solutions
The solutions which obey raoult’s law at all compositions of solute in solvent at all temperature are called ideal solution.
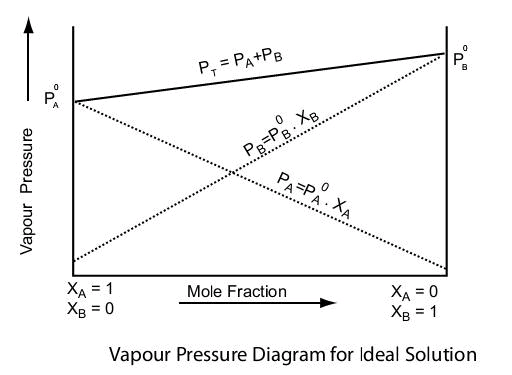
Ideal solution can be obtained by mixing two components with identical molecular size, structure and they should have almost same intermolecular attraction e.g., two liquids A and B form an ideal solution when A-A and B-B molecular attractions will be same, and hence A-B molecular attraction will be almost same as A-A and B-B molecular attractions.
An ideal solution should have following characteristics
1. It should obey Raoult’s law i.e., PA = XAP°A and PA = XBP°B
2. ΔHmixing = 0, i.e. no heat should be absorbed or evolved during mixing.
3. ΔHmixing = 0, i.e. no expansion of contraction on mixing.
Examples of Ideal solutions:
(i) Ethyl chloride and ethyl bromide
(ii) n-hexane and n-heptane
(iii) CCl4 and SiCl4
.
Non-ideal Solutions
The solution which deviates from ideal behaviour are called nonideal solution or real solutions and they do not obey Raoult’s law over entire range of composition. It has been found that on increasing dilution, a nonideal solution tend to be ideal.
Many pairs of liquids are present in which there is no uniformity of attractive forces, i.e., the adhesive and cohesive forces of attraction are not uniform between the two liquids, so that they deviate from the Raoult's law applied only to ideal solutions.
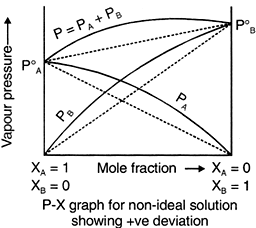
For non ideal solutions,
(i) 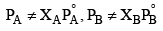 i.e. they do not obey Raoult's law
i.e. they do not obey Raoult's law
(ii) ΔHmixing ≠ 0
(iii) ΔVmixing ≠ 0
Here, we have two cases:
Positive Deviation
When (i) PA > XA P°A & PB > XBP°B
(ii) ΔHmix > 0,
(iii) ΔVmix > 0
When the cohesive forces between like molecules are greater than the adhesive forces between dissimilar molecules, the dissimilarities of polarity leads both components to escape solution more easily. Therefore, the vapor pressure is greater than expected from the Raoult's law, showing positive deviation. If the deviation is large, then the vapor pressure curve shows a maximum at a particular composition and form a positive azeotrope. Some mixtures in which this happens are
(1) benzene and methanol, (2) carbon disulfide and acetone, and (3) chloroform and ethanol. When these pairs of components are mixed, the process is endothermic reaction as weaker intermolecular forces are formed so that ΔmixH is positive.
Negative Deviation
If the vapor pressure of a mixture is lower than expected from Raoult's law, there is said to be a negative deviation.
(i) PA < XAP°A & PB < XBP°B
(ii) ΔHmix < 0
(iii) ΔVmix < 0
- Negative deviations from Raoult's law arise when the forces between the particles in the mixture are stronger than the mean of the forces between the particles in the pure liquids.
- This is evidence that the adhesive forces between different components are stronger than the average cohesive forces between like components.
- In consequence, each component is retained in the liquid phase by attractive forces that are stronger than in the pure liquid so that its partial vapor pressure is lower.
- For example, the system of chloroform (H2O) and acetone (CH3COCH3) has a negative deviation from Raoult's law, indicating an attractive interaction between the two components that has been described as a hydrogen bond.
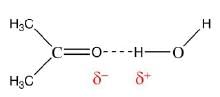
- The system hydrochloric acid - water has a large enough negative deviation to form a minimum in the vapor pressure curve known as a (negative) azeotrope, corresponding to a mixture that evaporates without change of composition.
- When these two components are mixed, the reaction is exothermic as ionic bonds H3O+— Cl– are formed so that ΔHmix is negative.
|
90 videos|144 docs|67 tests
|
FAQs on Introduction to Solutions, Ideal & Non Ideal Solutions - Physical Chemistry
| 1. What are the different types of solutions? |  |
| 2. What is the vapour pressure of a solution? |  |
| 3. What is Raoult's Law? |  |
| 4. How is the composition of the vapour determined in a solution? |  |
| 5. What are ideal and non-ideal solutions? |  |
















