Chemical & Physical Properties of Alkaline Earth Metals | Inorganic Chemistry PDF Download
Group 2 Elements (Alkaline Earth metals)
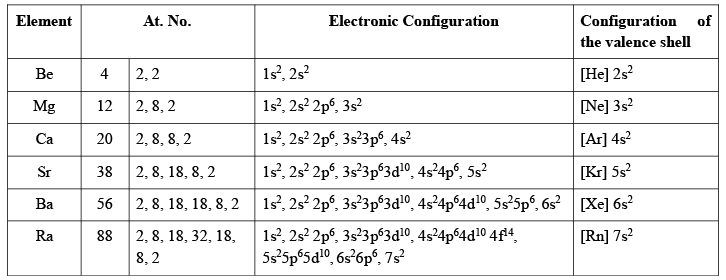
Electronic Configuration
The arrangement of the distribution of electrons on various subshells in the atoms of alkaline earth metals is given below:
Physical Properties
1. Physical State
They are all silvery white metals. They have grayish white lustre when freshly cut, but tarnish soon after their exposure in air due to surface oxidation.
2. Atomic and ionic radii
The size of the atom increases gradually from Be to Ra, on account of the presence of an extra energy shell at each step.
3. Density
These metals are dense than alkali metals in the same period because these can be packed more tightly due to their greater nuclear charge and smaller size. The density decreases slightly upto calcium and their increases considerably upto radium. Irregular trend is due to the difference in the crystals structure of these elements.
4. Melting and boiling points
The milting and boiling of these elements are higher than corresponding alk ali metals. This id due to the presence of two electrons in the valency shell the thus, strongly bonded in the solid state. However melting and boiling points do not show any regular trend because atoms adopt different crystal structures.
Be > Mg > Ca > Sr > Ba > Ra
5. Ionization energies and electropositive character
The first and second ionization energies of these metals decreases form Be to Ba. The second ionization energy in each case is higher than the first, nearly double the first ionization energy.
6. Oxidation States All show a stable oxidation state +2 in their compounds. The second ionization energy is nearly dou ble the first ionization energy for all these elements. This should cau se these elements to exhibit a stable +1 oxidation state and form compounds like BaCl, SrBr, Cal, etc, instead of BaCl2, SrBr2, Cal2, etc.
- Amongst alkaline earth metals, beryllium has the highest ionization energy, i.e., least electropositi ve in nature.
- Thus, beryllium has minimum tendency to form Be2+ ion and hence a number of compounds of beryllium are covalent in nature.
7. Hydration of ions and hydration energy
The M2+ ions of alkaline earth metals are extensively hydrated to form hydrated ions [M(H2O)x]2+ and during hydration a huge amount to energy, called hydration energy, is released.
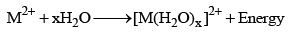
The degree of hydration and the amount of hydration energy decreases as the size of the ion increases from Be2+ to Ba2+
The hydration energies of alkaline earth metal ions are higher than those of alkali metal ions and thus the compounds of alkaline earth metals are more extensively hydrated than alkali metals,
Magnesium chloride and calcium chloride exist as MgCl2.6H2O and CaCl2.6H2O, respectively, while sodium chloride and potassium chloride exists as NaCl and KCl.
- The ionic mobilities or ionic condu ctance of these ions increase from [Be(H 2O)x]2+ due to high degree to hydration.
8. Flame coloration
In beryllium and magnesium, the electrons are tightly held and hence excitation is rather difficult, thus do not show flame coloration. Ca, Sr, Ba and Ra impart a characteristic colour to the flame.
Ca-brick red Sr-crimson Ba-green Ra-crimson
9. Reducing nature
The alkaline earth metals have the tendency to lose electrons and change into bivalent cation: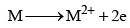
Hence, they act as strong reducing agents. The reducing nature increases as the atomic number increases. Strength of a reducing agent is linked with the value of oxidation potential. The values of the oxidation potentials increases from Be to Ba, hence the strength as a reducing agent increases in the same order.
Be | Mg | Ca | Sr | Ba | |
Oxidatio n Potential (Volt) | 1.85 | 2.37 | 2.87 | 2.89 | 2.90 |
The oxidation potentials are lower than those of the alkali metals, hence, the alkaline earth metals are weaker reducing agents than alkali metals.
- The reason for the lower values of oxidation potentials is du e to high heats of atomiza tion (sublimiation) a nd ionization energies.
10. Colour and magnetic property
Since, the divalentions have noble gas configuration with no unpaired electrons, their compounds are diamagnetic and colourless unless the anion is coloured. The metals are also diamagnetic in nature as all the orbitals are fully filled with spin paired electrons, e.g.
Mg = 2, 8
= 1s2, 2s2 2p6

CHEMICAL PROPERTIES
The following are important minerals of these elements.
1. | Berylium | Beryl | 3BeO, Al2O3, 6SiO2 |
2. | Magnesium | Magnetic | MgCO3 |
Dolomite | MgCO3, CaCO3 | ||
Carnallite | KCl. MgCl2. 6H2O | ||
Epsomite or Epsom salt | MgSO4. 7H2O | ||
Asbestos | CaMg3(SiO3)4 | ||
3. | Calcium | Lime, stone, chalk | CaCO3 |
Calcite, marble | CaCO3 | ||
And Iceland spar | CaCO3 | ||
Gypsum | CaSO4.2H2O | ||
Fluorspar | CaF2 | ||
4. | Strontium | Celestine | SrSO4 |
Strontianite | SrCO3 | ||
5. | Barium | Barytes or heavy spar | BaSO4 |
Witherite | BaCO3 | ||
6. | Radium | Pitchblende, Carnotite | Minerals of uranium consisting mineutes quantites of radium. |
1. Action of water
Calcium, strontium, barium and radium decompose cold water readily with evolution of hydrogen
M + 2H2O → M(OH)2 + H2
2. Action of atmosphere
Except beryllium, these metals are easily tarnished in ai4r as a layer of oxide is formed on their surface. The effect of atmosphere increases as the atomic number increase. Barium in powdered form bursts into flame on exposure to air.
3. Action of acids
Like alkali metals, the alkaline earth metals freely react with acids and displace hydrogen.
M + H2SO4 → MSO4 + H2
Beryllium behaves differently as it dissolves in caustic alkalies also with liberation of hydrogen. It is due to diagonal relationship with alu minium.
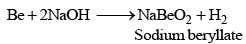
4. Reaction with hydrogen
Expect beryllium, all combine with hydrogen directly to form hydrides of the type MH2 when heated with hydrogen.
M + H2 → MH2
BeH2 and MgH2 are covalent in nature while other hydrides are ionic in nature. Calcium, strontiu m and barium hydrides liberate hydrogen at anode on electrolysis in the fused state. Ionic hydrides are violently decomposed by water evolving hydrogen. CaH2 is technically called hydrolith and used on large scale for the production of hydrogen.
CaH2 + 2H2O → Ca(OH)2 + 2H2
[BeH2 is not obtained by direct combination of beryllium and hydrogen. It is foemd by reacting beryllium chloride with lithium aluminium hydride.
2BeCl2 + LiAH4 → 2BeH2 + LiCl + AlCl3
It is polymeric (BeH2)n and possesses hydrogen bridges.
Three centre bonds are present in which a banana shaped molecular orbital covers three atoms Be----H-----Be and contains two electrons. Hydrogen atoms lie in the plane perpendicular to the plane of molecule containing beryllium atoms.
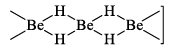
The stability of the hydrides decreases with increasing atomic number because the metallic nature of the elements increases.
5. Reaction with oxygen (Oxides and Hydroxides)
Except Ba and Ra, these elements when burnt in oxygen form oxides of the type MO.
2M + O2 → 2MO
Berylium metal is relatively unreactive and does not react burns brilliantly. The element, Mg burns with dazzling brilliance evolving a lot of heat.
Berium and radium, being highly electropositive, form peroxides.
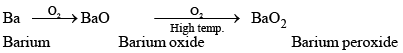
Thus, the affinity for oxygen increases on moving down the group.
BeO is usually formed by ignition of the metal but the other metal oxides (MO type) are usually obtained by thermal decomposition of the carbonates, MCO3
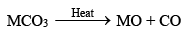
The oxides are very stable compounds (BeO and MgO are used as refractory materials) and white crystalline solids.
BeO is covalent and has a 4:4 zinc sulphide (wurtzite) structure. All the other oxides are ionic and possess 6:6 NaCl structure (face centred cubic). The reason for high stability is due to high lattice energy values which, however, decreases as the size of the metal ion increases.
Except BeO, which is amphoteric in nature, other MO oxides are basic in nature as they combine with water to form basic hydroxides. This reaction is highly exothermic.
MO + Heat → M(OH)2 + Heat
(where, M = Ca2 + Sr2+ or Ba2+)
Basic nature of the oxides increases gradually from BeO to BaO
6. Reaction with halogens (Halides)
The alkaline earth metals directly combine with halogens, when heated with them.
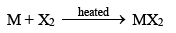
Beryllium halides are covalent in nature. This is due to small size and high charge of Be2+ ion, i.e. it has high polarizing power. The glassy forms of halides are known to have chains of -------X2BeX2Be------
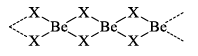
7. Action with nitrogen
All the alkaline earth metals burn in nitrogen to form nitrides of the type
M3N2. 3M + N2 → M3N2
The ease of formation of nitrides decreases from Be to Ba.
8. Action with carbon (Carbides)
With the exception of Be, other metals when heated with carbon form carbides of the type MC2. These carbides are called acetylides as on hydrolysis they evolve acetylene.
M + 2C → MC2
MC2 + 2H2O → M(OH)2 + C2H2 Acetylene
- CaC2 is an important chemical intermediate. When CaC2 is heated in an electric furnace with atmospheric dinitrogen at 1100°C, calcium cyanamide CaNCN is formed.
- The cyanamide ion [N = C= N]2- is isoelectronic with CO2, and has the same linear stru cture.
CaNCN + H2SO4 → H2NCN + CaSO4
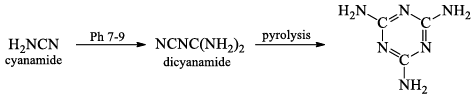
9. Nature of oxysalts
(i) Bicarbonates and carbonates
Bicarbonates of alkaline earth metals do not exist in solid state but are known in solutions only. When such solutions are heated, bicarbonates are decomposed with evolution of carbon dioxide.
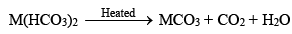
Carbonates of alkaline earth metals (MCO3) are insoluble in water. These dissolve in water in presence of carbon dioxide.
MCO3 + H2O + CO2 → M(HCO3)2
Solubility of carbonates decreases on moving down the group, while stability increases. This is evident from the values of decomposition temperatures of various carbonates which increases gradually.
Increasing stability can be explained on the basis of polarization and covalent character. Be2+ is smallest in size hence show high polarizing power. BeCO3 is least ionic and has least stability.
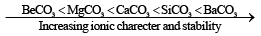
- The carbonates are all ionic, but BeCO3 is unusual because it contains hydrated ion [Be(H2O)4]2+ rather than Be2+
(ii) Sulphates
Alkaline earth metals form sulphates of the type MSO4. These are prepared by the action of sulphuric acid on oxides, hydroxides or carbonates.
MO + H2SO4 → MSO4 + H2O
The solubility of sulphates decreases on moving down the group. CaSO4 is springly solu ble, while SrSO4, BaS4 and RaSO4 are almost insolu ble. The solubilities of BeSO4 and MgSO4 are due to high energy of solvation of smaller Be2+ and Mg2+ ions.
The sulphates decomposes on heating to give the corresponding oxide (MO).
The stability increases as the basic nature of the metal increases.
Difference Between Alkaline Earth Metals and Alkali Metals
Properties | Alkaline earth metals | Alkali metals |
(i) electronic configuration | Two electrons are present in the valence shell. The configuration is ns2 | One electron is present in the valence shell. The configuration is ns1 |
(ii) Valency | Bivalent | Monovalent |
(iii) Electropositive nature | Less electropositive | More electropositive |
(iv) Hydroxides | Weak bases, less soluble and decompose on heating | Strong bases, highly soluble and stable towards heat |
(v) Bicarbonates | These are not known in free state. Exist only in solution | These are known in solid state. |
(vi) Carbonates | Insoluble in water. Decompose on heating | Soluble in water. Do not decompose on heating (Li2CO3 is an exception) |
(vii) Action of carbon | Directly combine will carbon and form carbides. | Do not directly combine with carbon. |
(viii) Action of nitrogen | Directly combine with nitrogen and form nitrides. | Do not directly combine with nitrogen |
(ix) Nitrates | Decomposes on heating evolving a mixture of NO2 and oxygen | Decompose on heating evolving only oxygen. |
(x) Solubility of salts | Sulphates, Phosphates, fluorides, Chromates, oxalates etc. are insoluble in water | Sulphates, phosphates, fluorides, chromates, oxalates, etc. are soluble in water |
(xi) Physical properties | Comparitively harder, High melting points, Diamagnetic | Soft, low melting points, Paramagnetic. |
(xii) Hydration of Compounds | The compounds are extensively hydrated. MgCl2.6H2O, CaCl2, 6H2O, BaCl2.2H2O are hydrated chlorides. | The compounds are less hydrated. NaCl, KCl, RbCl, form non-hydrated chlorides. |
(xiii) Reducing power | Weaker, as ionization potential values are high and oxidation potential values are low. | Stronger, as ionization potential values are low and oxidation potential values are high. |
Anomalous Behaviour of Beryllium
Be differs from the rest of the group for three reason.
(i) It is extremely small, and Faja ns, rules state that small highly charged ions to form covalent compounds.
(ii) Be has comparatively high electronegativity. Thu s when beryllium reacts with another atom, the difference in electronegativity is seldom large, which again favours the formation of covalent compounds. Even BeF2 (electronegativity difference 2.5) and BeO (electronegativity difference 2.0) show evidence of covalent character.
(iii) Be is in the second row of the periodic table, a nd the outer shell can hold a maximu m of eight electrons. (The orbitals available for bonding are one 2s and three 2p orbitals). Thus Be can form a maximum of four conventional electron pair bonds, and in many compounds the maximum coordination number of Be is 4. The later elements can have more than eight outer electrons, and may attain a coordination number of 6 using one s, three p and two d orbitals for bonding. Exceptions occur if multi-centre bonding occurs, as for example in basic beryllium acetate, when higher coordination number are obtained.
Thus we should expect Be to form mainly covalent compounds, and commonly have c coordination number of 4. Anhydrou s compou nds of Be are predominantly two -covalent, and BeX2 molecules should be linear.
In fact linear molecules exist only in the gas phase, as this electronic arrangement has not filled the outer shell of electrons. In the solid state four-fold coordination is always achieved. There are several ways by which this can be achieved.
(i) Two ligands that have a long pair of electrons may form coordinate bonds using the two unfiled orbitals in the valence shell of Be. Thus two F– ions might coordinate to BeF 2, forming [BeF4]2– Similarly diethyl ether can coordinate to Be(+II) in BeCl2, forming [BeCl2(OEt2)2].
(ii) The BeX2 molecules may polymerize to form chains, containing bridging halogen groups, for example (BeF2)n, (BeCl2)n. Each halogen forms one normal covalent bond, and uses a lone pair to form a coordinate bond.
(iii) (BeMe2)n has essentially the same stru cture as (BeCl2)n, but the bonding in the methyl compound is best regarded as three-centre two electron bonds covering one Me and two Be atoms.
(iv) A covalent lattice may be formed with a zinc blende or wurzite structure (coordination number 4).
For example by BeO and BeS.
In water beryllium salt are extensively hydrolyzed to give a series of hydroxo complexes of unknown structure. They may be polymeric and of the type.
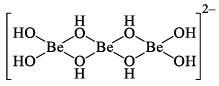
If alkali is added to these solutions the polymers break down to give the simple mononuclear beryllate ion [Be(OH)4]2–, which is tetrahedral. Many beryllium salts contain the hydrated ion [Be(H2O)4]2+ rather tha n Be2+ and the hydrated ion too is a tetrahedral complex ion. Note that the
coordination number is 4. Forming a hydrated complex increases the effective size of the berylliumion, thus spreading the charge over a larger area. Stable ionic salts such as [Be(H2O)4]SO4, [Be(H2O)4](NO3)2 and [Be(h2o)4]Cl2 are known.
Beryllium salts are acidic when dissolved in pure water because the hydrated ion hydrolyses, producing H3O+. This happens because the Be—O bond is very strong, and so in the hydrated ion this weakens the O—H bonds, and hence there is a tendency to lose protons. The initial reaction is
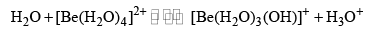
But this may be followed by further polymerization, involving hydroxobridged structures [Be2OH]3+, [Be3(OH)4]3+. In alkaline solutions [Be(OH)4]2– is formed. The other Group 2 salts do not interact so strongly with water, and do not hydrolyse appreciably.
Beryllium salts rarely ha ve more than four molecules of water of crystallization a ssociated with the metal ion, because there are only four orbitals available in the second shell of electrons, whereas magnesium can have a coordination number of 6 by using some 3d orbitals as well as 2s and 3p orbitals.
SIMILARITIES (DIAGONAL RELATIONSHIP)
BETWEREN BERYLLIUM AND ALUMIMIUM
Beryllium shows some similarities in properties with aluminium, the second typical element of group IIA (next group in Mendeleefs periodic table) of the next higher period. This type of relationship between diagonally placed elements is called diagonal relationship.
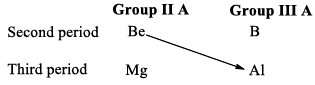
|
48 videos|92 docs|41 tests
|
FAQs on Chemical & Physical Properties of Alkaline Earth Metals - Inorganic Chemistry
| 1. What are the chemical properties of alkaline earth metals? |  |
| 2. What are the physical properties of alkaline earth metals? |  |
| 3. How do alkaline earth metals react with water? |  |
| 4. What are the uses of alkaline earth metals? |  |
| 5. Why are alkaline earth metals considered important in biological systems? |  |

|
Explore Courses for Chemistry exam
|

|


















