Class 9 Exam > Class 9 Notes > Osmosis experiment with egg lab report for biology
Osmosis experiment with egg lab report for biology - Class 9 PDF Download
How does osmosis keep you healthy?
Right now, as you read this, there are millions of things happening throughout your body. The food you ate just a bit ago is making its way through a watery slurry inside your stomach and small intestines. Your kidneys are working hard to excrete waste and extra water. The lacrimal glands near your eyes are secreting tears, which allow your eyelids to close without damaging your eyeballs. What’s one thing that all of these processes have in common? They all rely on osmosis: the diffusion of water from one place to another.
Osmosis factors heavily in each of these processes and is an important force for keeping every single cell in your body healthy. Osmosis is hard to see without a microscope. But if we create our very own model of a cell, using a shell-less chicken egg, we can see what happens when we manipulate the osmotic balance in the “cell”!
Materials
3 eggs
3 glasses (large enough to fit the egg plus liquid)
3 butter knives
White vinegar (about 3 cups)
Distilled water (about 2 cups)
Light corn syrup (about 1 ¼ cups)
Slotted spoon
Measuring cup (1 cup)
Measuring spoons (1 tablespoon and ½ tablespoon)
Sticky notes and marker
Scale (optional)
Procedure
Note: It’s okay to touch the eggs, but remember to wash your hands afterwards to avoid any nasty surprises!
Place one egg in each glass. Pour in enough vinegar to cover each egg. Bubbles will start to form around the egg, and it’ll float up. To keep it submerged, put a butter knife in the glass to hold it down.
Put the three glasses in the refrigerator and allow to sit for 24 hours.
Gently holding the egg in the glass, pour out the old vinegar. Replace with fresh vinegar, and let sit in the refrigerator for another 24 hours. Repeat this process until the shells are fully dissolved and only the membrane remains. This should take about 2-3 days.
Gently remove the eggs using the slotted spoon and rinse with tap water in the sink. Rinse out the empty glasses as well.
Gently put the shell-less eggs aside for a moment on a plate.
Prepare three different sugar-water solutions as follows, labeling with sticky notes:
Glass 1: Label “hypertonic”. Pour in one cup of corn syrup.
Glass 2: Label “isotonic”. Add 1 ½ tablespoons corn syrup to the one cup measuring cup, and fill the remainder with distilled water. Pour into glass (make sure you get all the corn syrup out!) and stir to dissolve.
Glass 3: Label “hypotonic”. Pour in one cup of distilled water.Gently put one shell-less egg in each of the glasses, and let sit in the refrigerator for another 24 hours.
Remove the glasses from the refrigerator, and gently put the eggs on a plate. If you weighed the eggs before putting them in each solution, weigh them again. What happened to each of the eggs?
How does osmosis work?
Osmosis is the scientific term that describes how water flows to different places depending on certain conditions. In this case, water moves around to different areas based on a concentration gradient, i.e. solutions which have different concentrations of dissolved particles (solutes) in them. Water always flows to the area with the most dissolved solutes, so that in the end both solutions have an equal concentration of solutes. Think about if you added a drop of food dye to a cup of water – even if you didn’t stir it, it would eventually dissolve on its own into the water.
In biological systems, the different solutions are usually separated by a semipermeable membrane, like cell membranes or kidney tubules. These act sort of like a net that keeps solutes trapped, but they still allow water to pass through freely. In this way, cells can keep all of their “guts” contained but still exchange water.
Now, think about the inside of an egg. There’s a lot of water inside of the egg, but a lot of other things (i.e. solutes) too, like protein and fat. When you placed the egg in the three solutions, how do you think the concentration of solutes differed between the inside of the egg and outside of the egg? The egg membrane acts as a semipermeable membrane and keeps all of the dissolved solutes separated but allows the water to pass through.
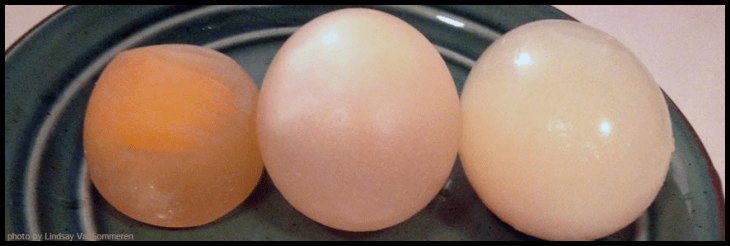
How did osmosis make the eggs change size (or not)?
If the steps above work out properly, the results should be as follows.
In the case of the hypertonic solution, there were more solutes in the corn syrup than there were in the egg. So, water flowed out of the egg and into the corn syrup, and as a result the egg shriveled up.
In the case of the isotonic solution, there was roughly an equal amount of solutes in the corn syrup/water solution than there was in the egg, so there was no net movement in or out of the egg. It stayed the same size.
In the case of the hypotonic solution, there were more solutes in the egg than in the pure water. So, water flowed into the egg, and as a result, it grew in size.
FAQs on Osmosis experiment with egg lab report for biology - Class 9
| 1. What is osmosis? |  |
Ans. Osmosis is the process by which water molecules move from an area of low solute concentration to an area of high solute concentration across a selectively permeable membrane. It plays a crucial role in maintaining the balance of water and solutes in living organisms.
| 2. How does osmosis occur in the egg experiment? |  |
Ans. In the egg osmosis experiment, a shell-less egg is placed in different solutions (such as water, saltwater, or sugar solution) to observe the movement of water through the egg's membrane. The egg's membrane acts as the selectively permeable membrane, allowing water molecules to pass through while restricting the passage of solutes. Osmosis occurs as water molecules move from the solution with lower solute concentration to the solution with higher solute concentration, resulting in the change in the egg's size.
| 3. What factors affect the rate of osmosis in the egg experiment? |  |
Ans. Several factors can affect the rate of osmosis in the egg experiment. These include the concentration gradient between the egg and the surrounding solution, the temperature of the solutions, the surface area of the egg, and the duration of the experiment. A steeper concentration gradient, higher temperature, larger surface area, and longer duration can generally increase the rate of osmosis.
| 4. What can be inferred from the changes in the egg's size during the osmosis experiment? |  |
Ans. The changes in the egg's size during the osmosis experiment provide insights into the movement of water across the egg's membrane. If the egg increases in size, it indicates that water has moved into the egg, suggesting that the surrounding solution had a lower solute concentration than the egg. Conversely, if the egg decreases in size, it indicates that water has moved out of the egg, suggesting that the surrounding solution had a higher solute concentration than the egg.
| 5. How can the osmosis experiment with eggs be related to real-life scenarios? |  |
Ans. The osmosis experiment with eggs can be related to real-life scenarios such as the process of food preservation. For example, when food is preserved using salt or sugar, it creates a high solute concentration around the food, causing water to move out of the food through osmosis. This helps to prevent the growth of bacteria and other microorganisms that require water to survive. Similarly, understanding osmosis is also important in understanding how cells regulate their water balance and how plants absorb water from the soil.
Download as PDF

|
Explore Courses for Class 9 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
Related Searches

















