Class 9 Science: Sample Question Paper- 12 (With Solutions) PDF Download
SECTION - A
Q.1. In adjoining figure displacement-time graphs for two particles A and B have been shown. Select the only wrong statement out of the following:
(а) Both A and B are describing linear paths.
(b) Both A and B are moving with uniform velocity.
(c) Velocity of A is more than that of B.
(d) Velocity of B is more than that of A.
Ans: (c) The only wrong statement is “velocity of A is more than that of B ”. Here both the motions are uniform linear motions. Moreover velocity of B is more because x-t graph of B has a greater slope.
Q.2. If an atom contains one electron and one proton, will it carry any charge or not ? .
Ans: It will not carry any charge because the positive charge on the proton neutralise the negative charge on the electron.
Q.3. Mass of one atom of oxygen is
(a) 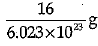
(b) 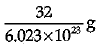
(c) 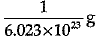
(d) 8u
Ans: (a)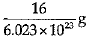
Explanation: Mass of 6.023 X 1023 atoms of Oxygen = Gram atomic mass of Oxygen Mass of 6.023 x 1023 atoms = 16 g.
Mass of 1 atom of oxygen = 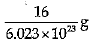
Q.4. A sonar sends a pulse of frequency 500 Hz towards the bottom of a lake.
The echo is received after 0.4 s. The speed of waves in water is 1450 m s-1. The depth of the lake is
(a) 200 m
(b) 1000 m
(c) 290 m
(d) 580 m
Ans: (c) Speed of sound waves in water v = 1450 m s-1 and time of echo t = 0.4 s
∴ Depth of lake d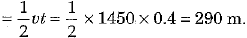
Q.5. The unit of relative density is:
(a) kg m-3
(b) g cm-3
(c) g litre-1
(d) it does not have a unit
Or
Two balls, one of iron and other of aluminium experience same upthrust when dipped in water,
(a) both have equal volume
(b) both have equal weight in air
(c) both have equal density
(d) nothing definite can be said
Ans: (d) Or (a)
Q.6. Rusting of an article made up of iron is called
(a) corrosion and it is a physical as well as chemical change
(b) dissolution and it is a physical change
(c) corrosion and it is a chemical change
(d) dissolution and it is a chemical change
Ans: (c) corrosion and it is a chemical change
Explanation: Rusting of an article is a corrosion and it is a chemical change, In the presence of water or air moisture, the redox reaction of iron and oxygen occurs which forms iron oxide, usually red oxide called as rust.
Q.7. Which of the following ions have a valency of + 3 or - 3 ?
(a) Phosphate
(b) Sulphate
(c) Barium
(d) Aluminium
Ans: (а) and (d)
Q.8. Which of the following is a recently originated problem of environment ?
(a) Ozone layer depletion
(b) Greenhouse effect
(c) Global warming
(d) All of these
Or
Marked temperature changes in aquatic environment can affect:
(a) Breeding of animals
(b) More growth of aquatic plants
(c) Process of digestion in animals
(d) Availability of nutrients
Ans: (d) Or (a)
Q.9. Who introduced the system of scientific nomenclature of organism?
(a) Robert Whittaker
(b) Carl Linnaeus
(c) Robert Hooke
(d) Ernst Haeckel
OR
Elephantiasis is caused by
(a) Wuchereria
(b) Pinworm
(c) Planarians
(d) Liver flukes
Ans: (b) Carl Linnaeus
Explanation: Carl Linnaeus formulated the method of naming organisms called the binomial system of nomenclature. In this system/ each type of organism has two Latin names i.ev one for its genus anc the other for its species.
OR
(a) Wuchereria
Explanation: Elephantiasis is caused by nematode parasites Wuchereria bancrofti. It is a condition (alsc called filariasis) in which a limb or other part of the body becomes enlarged due to blockage of tlu lymphatic vessels.
Q.10. If there were no atmosphere around earth, the temperature of the earth will
(а) increase.
(b) go on decreasing.
(c) increase during the day and decrease during the night. id) be unaffected.
Ans: (c) In the absence of atmosphere around the earth, the temperature of the earth will increase during the day and decrease during night.
Q.11. The molecular formula faluminium sulphate is:
(a) Al2(SO4)3
(b) AlSO4
(c) Al3(SO4)2
(d) Al2(SO4)4
Ans: (a)
Q.12. What is the duration of Kharif season ?
Ans: From the month of June to October.
Q.13. Kinetic energy is proportional to the mass and velocity of the moving object.
Ans: False - Kinetic energy is proportional to (i) mass (m)1 and (ii) square of the velocity (v2) of the object.
Q.14. Assertion: The value of acceleration due to gravity (g) is independent of the mass of the body.
Reason: The value of acceleration due to gravity g = 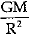 , where M is mass of planet (earth) and R is radius of planet (earth).
, where M is mass of planet (earth) and R is radius of planet (earth).
(i) A
(ii) B
(iii) C
(iv) D
Ans: (i)
SECTION - B
Q.15. (a) Given below are SI units of some physical quantities:
kg m s-1, m s-1 kg m s-2, m s-2
Which one of the above can be used for measuring momentum of an object ?Write the physical quantity that needs to be varied if the momentum of a body of given mass has to be changed.
(b) A constant force acts on an object of mass 2 kg for 10 s and increases its velocity from 5 m s-1 to 10 m s-1. Find the magnitude of applied force. If this force was applied for a duration of 15 s, what would be the velocity of the object ?
Ans: (a) The unit kg m s-1 measures momentum of an object. To change the momentum of a body of given mass we must change its velocity because momentum is given by the product of mass and velocity.
(b) Here mass m = 2 kg, initial velocity u = 5 m s-1 and final velocity v = 10 m s-1 after a time t = 10 s
∴ Acceleration a = 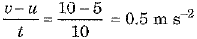
∴ Force F = ma = 2 x 0.5 = 1 N
Further velocity v' after a time t' - 15 s will be:
v' - u + at' = 5 + 0.5 x 15 = 12.5 m s-1
Q.16. (a) What is the effect of temperature on the solubility of a solid in liquid?
(b) Why is it possible to distinguish the particles of solution from those of solvent in a suspension ?
Or
When a bar magnet is brought closer to the mixture of iron filings and sulphur powder. Iron particles stick to the magnet, while incase of iron sulphide, no change will be observed. When a mixture of iron and sulphur is added to carbon disulphide, sulphur dissolves while incase of iron sulphide, no change is observed.
Ans: (a) If temperature increases, solubility increases and if temperature decreases, solubility decreases.
(b) Because particle size is bigger in suspensions.
Or
Properties of a compound are different from its constituents, while a mixture shows the properties of its constituent elements. Justify this statement taking the example of iron and sulphur.
Q.17. (a) State any one difference between pure and impure substances.
(b) What will you observe when:
(i) Carbon disulphide is added to the compound iron sulphide ?
(ii) Dil. hydrochloric acid is added to a mixture of iron filings and sulphur powder.
OR
Draw a flowchart showing the separation of components of air. Also, name this process.
Ans: (a) Pure substances contain only one kind of particles or two or more particles in a fixed ratio while impure substances contain more than one kind of particles.
(b) (i) No reaction,
(ii) Evolution of gas
OR
Air is a homogeneous mixture and can be separated into its components by the process of fractional distillation. The flow diagram shows the steps of the process :
Q.18. List two differences between a pure substance and a mixture. Give one example of each.
Or
Give the classification of elements with examples.
Ans: A pure substance consists of one type of particles while a mixture consists of different types of particles. A pure substance cannot be separated into other kinds of matter while a mixture can be separated into different kinds of matter. Sodium chloride and water are separately pure substances but sodium chloride solution is a mixture.
OR
Elements are classified into metals, non-metals and metalloids.
Examples: Metals: Sodium, potassium, calcium, copper, iron, zinc.
Non-metals: Hydrogen, carbon, oxygen, sulphur, chlorine.
Metalloids: Boron, silicon, arsenic.
Q.19 (a) Draw and identify different elements of phloem.
(b) Name the muscular tissues which is present in the iris of the eye. What is the shape of these cells ?
Ans: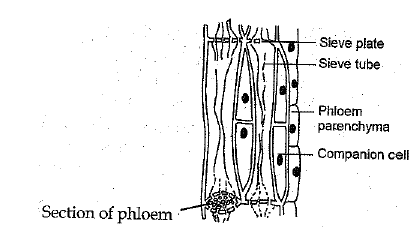
(a) Different elements of phloem are :
Sieve tubes, companion cells, phloem fibres and phloem parenchyma.
(b) (i) Unstriated/ smooth/ Involuntary
(ii) Spindle shaped.
Q.20. Write the chemical formulae of the following:
(i) Aluminium nitrate
(ii) Magnesium hydrogen carbonate
(iii) Sodium sulphate
OR
(i) Write the chemical formula of magnesium hydroxide.
(ii) Calculate the number of aluminium ions present in 0.051 g of aluminium oxide. [Atomic Mass of Al = 27 u]
Ans: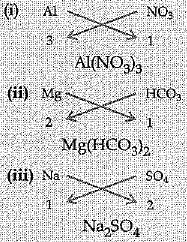
OR
(i) Mg(OH)2
(ii) Step I:
(Gram molecular mass of AI2O3)
= 2 x 27 + 3 x 16 = 102 g
∴ 102 g of Al2O3 = 1 mol
∴ 0.051 g of Al2O3 = 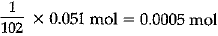
Step II:
1 mol. of Al2O3 contain Al atom = 2 x Avogadro No.
0.0005 eq. mol of Al2O3 contain Al atom
= 2 x 0.0005 x 6.022 x 1023
= 6.022 x 1020 atoms.
The number of Al ions (Al+3) present is the same as the number of Al atoms.
No. of Al3+ ions = 6.022 x 1020 ions.
Q.21. (a) Define Avogadro’s number.
(b) Show that 100 g of water and 100 g of carbon dioxide are not equal in number of moles. Molecular masses of water and carbon dioxide are IS u and 44 u respectively.
Ans: (a) Avogadro’s number is the number of particles, atoms, molecules or ions present in one mole of a substance. Its value is 6.022 x 1023.
(b) No. of moles of H20 = 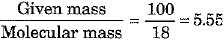
No. of moles of C02 = 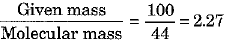
Thus, the number of moles in the two cases are not equal.
Q.22. Explain the following:
(i) Why it is difficult to walk on marshy land?
(ii) Why does an air filled balloon rise up slightly when punctured from below?
(iii) Why does a swimmer push water backward with his hands in order to swim in forward direction ?
Ans: (i) It is difficult to walk on marshy land because when we push the ground with our feet, the ground yields and gives no reaction.
(ii) As the air comes out of the punctured balloon it applies an upward reaction on the balloon. Thereby making it rise up slowly.
(iii) According to Newton's third law of motion, when the swimmer pushes the water backwards the water applies a forward reaction on him. This makes the swimmer move forward.
Q.23. Distinguish between density and relative density of a substance. The relative density of silver is 10.8. If the density of water is 103 kg/m3, find the density of silver.
Ans: Relative density measures the heaviness of a substance than the water. It is ratio of the density of a substance to that of water.
While density is mass per unit volume of a substance,
Relative density of silver = 10.8
Density of water = 1000 kg/m3
Density of silver = ?
Density of silver = R.D. x density of water.
= 10.8 x 1.000 kg/m3
= 10800 kg/m3
= 1.08 x 104 kg/m3.
Q.24. What factors may be responsible for losses of grains during storage ?
Ans: Food grains produced in our country are lost every year due to improper methods of storage and inadequate storage.
The various factors responsible for such loss during storage are:
(i) Biotic factors: They include insects, rodents, bacteria, fungi, mites, worms and birds.
(ii) Abiotic factors: They include inappropriate moisture, hum idity in air and temperature in the place of storage.
These factors cause poor quality, loss in weight, discolouration of produce, poor germinability and poor marketability of grains.
SECTION - C
Q.25. The velocity-time graph of an object is as shown below: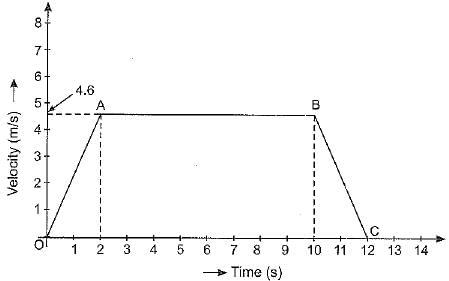
(а) Identify the kind of motion of the object represented by lines OA and BC.
(b) With what velocity the object is moving at t = 8 seconds ?
(c) Calculate the acceleration of the object in the following cases :
(i) Between the third and tenth second.
(ii) During the last two seconds.
Or
Define uniform circular motion. Is it an accelerated motion ? If yes, what is the direction of the acceleration ? Give an example of this type of motion.
Ans: (a) The line OA represents uniformly accelerated motion. The line BC represents uniformly retarded motion.
(b) At time t = 8 s, the object is moving with a velocity of 4.6 m s-1.
(c) (i) Between 3rd and 10th second, object is moving with a constant velocity of 4.6 m s-1. Hence, acceleration of object is zero.
(ii) During the last two seconds, velocity of object falls from u = 4.6 m s-1 to v = 0. Hence,
Acceleration = 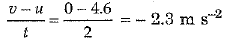
OR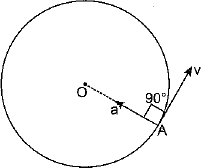
An object is said to be in uniform circular motion if it describes a circular path with a constant speed. Motion of the moon around the earth, motion of an electron around the nucleus in a circular orbit are some examples of uniform circular motion.
In uniform circular motion although speed of the moving particle remains constant, yet the direction of motion continuously changes with time so as to make its velocity variable Hence, uniform circular motion is an accelerated motion.
As the acceleration is changing only the direction of motion and the magnitude of the velocity (i.e., the speed) remains unchanged. It is possible only when the direction of acceleration is perpendicular to the direction of velocity. As being shown in figure, the direction of velocity at any point is along the tangent drawn on the circle at that point. Therefore, it is clear that direction of acceleration in uniform circular motion is along the radius of the circle and is pointed towards the centre of circle.
Q.26. a) Name the three subatomic particles of an atom.
b) Helium atom has an atomic mass of 4 u and two protons in its nucleus. How many neutrons it have ?
Ans: (a) (i) Electrons [negatively charged particles] which revolve around the nucleus.
(ii) Protons [positively charged particles] which are present in the nucleus.
(iii) Neutrons [having no charge] which are present in the nucleus.
(b) Mass number = Number of protons + Number of neutrons
4 = 2 + Number of neutrons
Number of neutrons = (4 - 2) = 2.
Q.27. 'Over crowded and poorly ventilated housing is a major factor in the spread of airborne diseases'. Explain the statement and support your answer with diagram also'.
Ans: (i) When we are closer to infected person, air transmitted diseases are easily transferred,
(ii) In closed areas, the droplet nuclei recirculate arid pose a risk to everybody.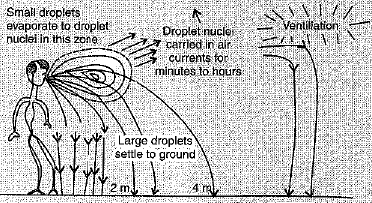
Q.28. Draw a plant cell and label the parts which
(а) determines the function and development of the cell.
(b) packages materials coming from the endoplasmic reticulum.
(c) provides resistance to microbes to withstand hypotonic external media without bursting.
(d) is site for many biochemical reactions necessary to sustain life.
(e) is a fluid contained inside the nucleus.
Or
The immune system of Hari is damaged by the attack of pathogen on his body.
(i) Name the disease he is suffering from.
(ii) Name the pathogen that has attacked his body.
(iii) Mention any three modes of transmission of this disease.
Ans: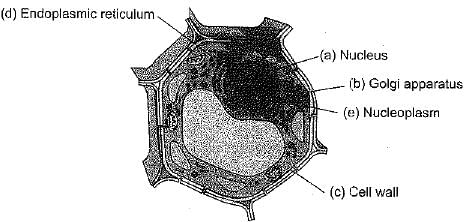
OR
(i) Hari is suffering from AIDS - Acquired Immuno Deficiency Syndrome.
(ii) The pathogen that has caused AIDS is HIV — Human Immuno Deficiency Virus.
(iii) This virus is transmitted through following ways :
(a) Sexual contact with an infected person carrying AIDS virus.
(b) Transfusion of blood infected with HIV.
(c) Use of unsterilised needles, blades or razors.
(d) Transplacental transmission i.e., AIDS infected mother to the foetus developing in her womb.
Q.29. (a) A cube of side 5 cm is immersed in water and then in saturated salt solution. In which case will it experience a greater buoyant force? If each side of the cube is reduced to 4 cm and then immersed in water, what will be the effect on the buoyant force experienced by the cube as compared to the first case for water? Give reason for each case.
(b) A ball weighing 4 kg of density 4000 kg m-3 is completely immersed in water of density if f kg m-3.
Find the force of buoyancy on it.
(Given g = 10 m s-2)
Ans: (a) Buoyant force = Vρg. Since density (ρ) of saturated salt solution is more than that of water, so the cube will experience greater buoyant force in saturated salt solution when size of cube is reduced, its volume (V) also reduces. Hence, it will experience less buoyant force than in first case.
(b) Buoyant Force = Vρg = volume of water displaced x density of water x g= mass of ball/density of ball x density of water x g = 4/4000 x 103 x 10 = 10 N
Q.30. Do all cells of our body look alike in terms of shape, size and structure ? What similarities do they have ? Illustrate by drawing diagrams of various cells present in human body.
OR
(a) What is the name given to the thread shaped structures in the nucleus ? Why is it important?
(b) Draw a diagram of the nudeus to show, the given parts
(i) nucleolus,
(ii) nuclear pore,
(iii) nuclear envelope.
Ans: No, not all cells in our body look alike in terms of shape, size and structure. The shape, size and structure of cell are determined by the function which it performs.
The eggs of many animals are spherical in shape, some are oval in shape, smooth muscle fibres are s- spindle shaped, nerve cells are elongated and RBCs arc discoidal in shape. The size and number of cells also vary from organism to organism.
Similarities : All cells have the same organelles. 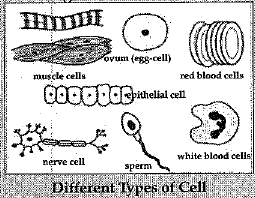
OR
(a) The thread shaped structures in the nucleus are known as chromosomes. These are important because they contain information for inheritance of features from parents to the next generation.
(b)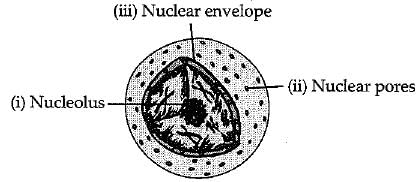
FAQs on Class 9 Science: Sample Question Paper- 12 (With Solutions)
| 1. What is the importance of solving sample question papers in Class 9 Science? |  |
| 2. How can solving sample question papers help in improving exam performance in Class 9 Science? |  |
| 3. Are the solutions provided in the sample question paper reliable for Class 9 Science? |  |
| 4. How can students effectively utilize sample question papers for Class 9 Science preparation? |  |
| 5. Can solving sample question papers alone guarantee success in the Class 9 Science exam? |  |


















