NEET Previous Year Questions (2014-2025): Chemical Kinetics | Chemistry Class 12 PDF Download
2025
Q1: If the rate constant of a reaction is 0.03s-1, how much time does it take for a 7.2 mol -1 concentration of the reactant to get reduced to 0.9 mol L-1 (Given: log 2=0.301) (NEET 2025)
(a) 210 s
(b) 21.0 s
(c) 69.3 s
(d) 23.1 s
Ans: (c)
Substitute the values into the formula:
t = - (1 / 0.03) × ln(0.9 / 7.2)
- Take the natural logarithm (ln):
ln(0.125) = -2.079 - Substitute into the equation:
t = - (1 / 0.03) × (-2.079) - Simplify:
t = (1 / 0.03) × 2.079
t = 69.3 s
Therefore, the time taken for the concentration to reduce from 7.2 mol L-1 to 0.9 mol L-1 is 69.3 seconds.
Q2: C(s) + 2H2(g) → CH4(g); ΔH = - 74.8kJmol-1
Which of the following diagrams gives an accurate representation of the above reaction?
[ R → reactants; P → products] (NEET 2025)
(a) 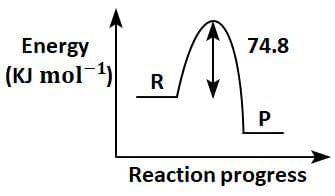
(b) 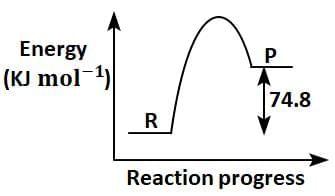
(c) 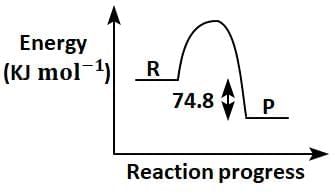
(d) 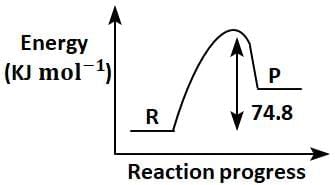
Ans: (c)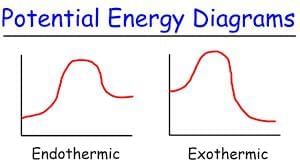
- In the given reaction:C(s) + 2H2(g) → CH4(g); ΔH = -74.8 kJ mol-1
- ΔH is negative, indicating that the reaction is exothermic.
- In an energy diagram for this reaction:
- The reactants (C and H2) start at a higher energy level.
- The products (CH4) are at a lower energy level, showing that energy is released during the reaction.
- The difference in energy between the reactants and products corresponds to the magnitude of ΔH (74.8 kJ mol-1).
Therefore, the correct energy diagram will show the reactants at a higher energy level than the products, with an energy drop of 74.8 kJ mol-1.
Q3: If the half-life (t1/2) for a first-order reaction is 1 minute, then the time required for 99.9% completion of the reaction is closest to: (NEET 2025)
(a) 5 minutes
(b) 10 minutes
(c) 2 minutes
(d) 4 minutes
Ans: (b)
From the formula for half-life:
t₁/₂ = 0.693 / k
- k = 0.693 / t₁/₂
- k = 0.693 / 1
- k = 0.693 min⁻¹
The time required for 99.9% completion can be calculated using the formula:
t = (2.303 / k) x log(1 / (1 - fraction completed))
- t = (2.303 / 0.693) x log(1 / (1 - 0.999))
- t = (2.303 / 0.693) x log(1 / 0.001)
- t = (2.303 / 0.693) x log(1000)
- t = (2.303 / 0.693) x 3
- t ≈ 10 minutes
Therefore, the time required for 99.9% completion of the reaction is approximately 10 minutes.
2024
Q1: Activation energy of any chemical reaction can be calculated if one knows the value of(a) rate constant at standard temperature
(b) probability of collision
(c) orientation of reactant molecules during collision
(d) rate constant at two different temperatures (NEET 2024)
Ans: (d)
To calculate the value of Ea
The equation used is
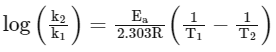
Hence, Ea can be calculated if the value of the rate constant k is known at two different temperatures, T1 and T2.
Q2: Which plot of In k vs 1 / T is consistent with the Arrhenius equation?
(a) 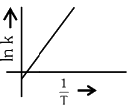
(b) 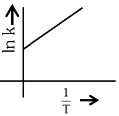
(c) 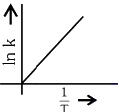
(d) 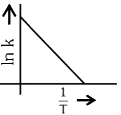 (NEET 2024)
(NEET 2024)
Ans: (d)
The Arrhenius equation is given as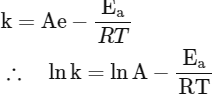
In k vs 1 / T gives a straight line graph with slope = 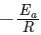 and intercept = InA
and intercept = InA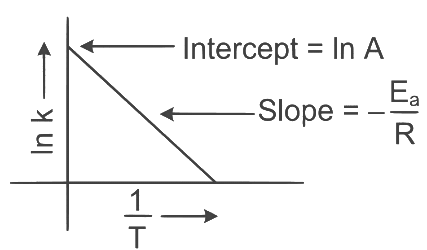
Q3: The rate of a reaction quadruples when temperature changes from 27∘C to 57∘C. Calculate the energy of activation.
Given R = 8.314 J K−1 mol−1,log4 = 0.6021
(a) 38.04 kJ/mol
(b) 380.4 kJ/mol
(c) 3.80 kJ/mol
(d) 3804 kJ/mol (NEET 2024)
Ans: (a)
To solve this problem, we will use the Arrhenius equation, which relates the rate constant (k) of a chemical reaction to the temperature (T) and the activation energy (Ea). The equation in its logarithmic form is: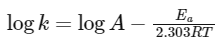
where:
- K is the rate constant,
- A is the pre-exponential factor (frequency factor),
- Ea is the activation energy,
- R is the universal gas constant
- T is the temperature in Kelvin,
According to the problem, the rate of the reaction quadruples (k2
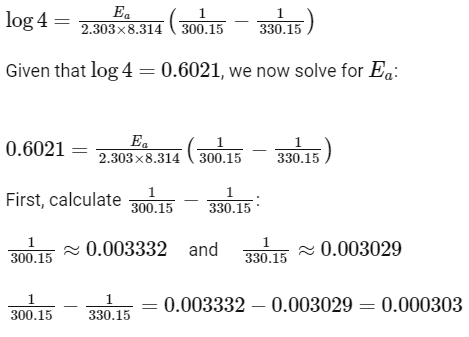
So,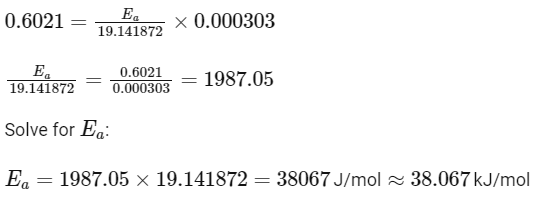
Therefore, the activation energy Ea is 38.07kJ/mol, which best matches Option A:
38.04kJ/mol
Q4: For the reaction:
2HI(g) → H₂(g) + I₂(g)
Which of the following rate expressions is correct? (NEET 2024)
(a) -Δ[HI] / Δt = 2Δ[H₂] / Δt
(b) -Δ[HI] / Δt = 4Δ[I₂] / Δt
(c) -Δ[HI] / Δt = Δ[H₂] / Δt
(d) Δt / Δ[HI] = Δt / Δ[H₂]
Ans: (a)
The given reaction is:
2HI(g) → H₂(g) + I₂(g)
The stoichiometric coefficients indicate the ratio of the change in concentration of each substance involved in the reaction.
- For every 2 moles of HI that are consumed, 1 mole of H₂ and 1 mole of I₂ are produced.
This gives us the following relationship for the rate of change of concentration:
- Δ[HI] is the concentration change of HI (reactant).
- Δ[H₂] is the concentration change of H₂ (product).
From the stoichiometry of the balanced equation, we know:
- For every 2 moles of HI consumed, 1 mole of H₂ is produced. Therefore, the rate of consumption of HI is twice the rate of formation of H₂.
Thus, the correct rate expression is: -Δ[HI] / Δt = 2Δ[H₂] / Δt
This matches with option (a).
Q5: Effective collisions are known to possess: (NEET 2024)
A: Energy greater than threshold energy.
B: Breaking of old bonds in reactants.
C: Formation of new bonds in products.
D: High activation energy.
E: Proper orientation.
Choose the correct answer from the options given below:
(a) A, B, C, D only
(b) A, B, C, E only
(c) A, C, D, E only
(d) B, C, D, E only
Ans: (b)
Effective collisions are those that lead to a chemical reaction. For a collision to be effective, the following conditions must be met:
- A: Energy greater than threshold energy: The colliding particles must have energy greater than the activation energy threshold for the reaction to occur.
- B: Breaking of old bond in reactant: During an effective collision, bonds in the reactant molecules must break to form products.
- C: Formation of new bond in product: New bonds must form in the product molecules as a result of the effective collision.
- E: Proper orientation: The molecules involved in the collision must be oriented in such a way that the reacting atoms or groups can interact effectively.
D: High activation energy is not necessary for an effective collision. It refers to the energy barrier the reactants need to overcome, but the effective collision occurs when the energy exceeds the threshold energy, not necessarily when the activation energy is high.
Thus, the correct answer is Option B: A, B, C, E only.
Q6: The time taken by the first-order decomposition of SO₂Cl₂ to decompose to 40% is 560 seconds. The rate constant for the reaction is: (Given: log 2.5 = 0.3979)
Choose the correct answer from the options given below:
(a) 5.476 × 10⁻² min⁻¹
(b) 4.876 × 10⁻² min⁻¹
(c) 5.176 × 10⁻² min⁻¹
(d) 4.576 × 10⁻² min⁻¹
Ans: (a)
For a first-order reaction, the rate constant (k) is related to the time taken to decompose a certain percentage of reactant using the following equation:
ln ([A]₀/[A]) = kt
Where:
- [A]₀ is the initial concentration,
- [A] is the concentration after time t,
- k is the rate constant,
- t is the time.
For first-order reactions, the equation becomes:
t = (1/k)*log ([A]₀/[A])
Given that 40% of SO₂Cl₂ decomposes, this means that 60% remains after 560 seconds.
So, [A] = 0.60[A]₀ and [A]₀/[A] = 1/0.60 = 1.6667.
Now, using the formula:
log (1.6667) = 0.2219 (logarithmic calculation).
Using this in the first-order equation:
log (1.6667) = kt / 2.303
0.2219 = k * 560 / 2.303
Solving for k:
k = (0.2219 * 2.303) / 560
k ≈ 0.0009127 s⁻¹
Convert to min⁻¹:
k ≈ 0.0009127 × 60 ≈ 0.05476 min⁻¹ = 5.476 × 10⁻² min⁻¹
Thus, the rate constant is 5.476 × 10⁻² min⁻¹, so the correct answer is (a).
Q7: The following data is for a reaction between reactants A and B: (NEET 2024) 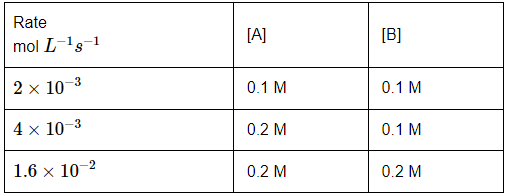 The order of the reaction with respect to A and B, respectively are:
The order of the reaction with respect to A and B, respectively are:
(a) 1, 0
(b) 0, 1
(c) 1, 2
(d) 2, 1
Ans: (c)
We can determine the order of the reaction with respect to A and B using the given data. The rate equation for a reaction is of the form:
Rate = k[A]m[B]n
Where:
- m is the order with respect to A,
- n is the order with respect to B,
- k is the rate constant.
We can calculate the orders with respect to A and B by comparing two experiments where one concentration is constant and the other changes.
Step 1: Find the order with respect to A (m):
- Compare experiments 1 and 2, where the concentration of B is constant (0.1 M) and the concentration of A changes from 0.1 M to 0.2 M.
- Rate 1 = 2 × 10⁻³, Rate 2 = 4 × 10⁻³.
- The ratio of the rates is:
- (Rate 2 / Rate 1) = (4 × 10⁻³) / (2 × 10⁻³) = 2.
- The ratio of the concentrations of A is:
- ([A]₂ / [A]₁) = (0.2 / 0.1) = 2.
- Since the rate doubles when the concentration of A doubles, the order with respect to A is 1.
Step 2: Find the order with respect to B (n):
- Compare experiments 2 and 3, where the concentration of A is constant (0.2 M) and the concentration of B changes from 0.1 M to 0.2 M.
- Rate 2 = 4 × 10⁻³, Rate 3 = 1.6 × 10⁻².
- The ratio of the rates is:
- (Rate 3 / Rate 2) = (1.6 × 10⁻²) / (4 × 10⁻³) = 4.
- The ratio of the concentrations of B is:
- ([B]₃ / [B]₂) = (0.2 / 0.1) = 2.
- The rate quadruples when the concentration of B doubles, which suggests the order with respect to B is 2.
Thus, the order of the reaction is 1 with respect to A and 2 with respect to B, so the correct answer is (c) 1, 2.
Q8: Which of the following plots represents the variation of lnk versus 1/T according to the Arrhenius equation? (NEET 2024)
(a) 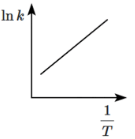
(b) 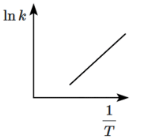
(c) 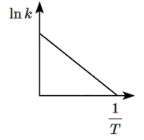
(d) 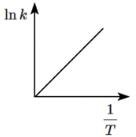
Ans: (c)
According to the Arrhenius equation:
ln k = -Ea/RT + ln A
Where:
- k is the rate constant,
- T is the temperature,
- Ea is the activation energy,
- R is the universal gas constant,
- A is the pre-exponential factor.
This equation shows that a plot of ln k versus 1/T should give a straight line with a slope of -Ea/R.
- The plot will have a negative slope because Ea is positive (activation energy is always positive).
- Therefore, the plot should be a downward-sloping line, which corresponds to option (c).
Thus, (c) is the correct plot that represents the variation of ln k with 1/T according to the Arrhenius equation.
Q9: Rate constants of a reaction at 500 K and 700 K are 0.04 s⁻¹ and 0.14 s⁻¹, respectively. The activation energy of the reaction is:
(Given: log 3.5 = 0.5441, R = 8.31 J K⁻¹ mol⁻¹) (NEET 2024)
(a) 182310 J
(b) 18500 J
(c) 18219 J
(d) 18030 J
Ans: (c)
We can use the Arrhenius equation to calculate the activation energy (Ea) of the reaction:
ln(k₂ / k₁) = (Ea / R) * (1/T₁ - 1/T₂)
Where:
- k₁ = 0.04 s⁻¹ (rate constant at T₁ = 500 K),
- k₂ = 0.14 s⁻¹ (rate constant at T₂ = 700 K),
- R = 8.31 J K⁻¹ mol⁻¹ (gas constant),
- T₁ = 500 K,
- T₂ = 700 K.
Now, substitute the known values into the equation:
ln(0.14 / 0.04) = (Ea/ 8.31) * (1/500 - 1/700)
First, calculate the ratio:
ln(0.14 / 0.04) = ln(3.5) = 0.5441
Now calculate the temperature difference:
(1/500 - 1/700) = (700 - 500) / (500 * 700) = 200 / 350000 = 0.0005714 K⁻¹
Now, substitute into the equation:
0.5441 = (Ea / 8.31) * 0.0005714
Solve for Ea:
Ea = (0.5441 * 8.31) / 0.0005714
Ea ≈ 18219 J
Therefore, the activation energy Ea is approximately 18219 J, which corresponds to option (c).
2023
Q1: For a certain reaction, the rate = k[A]2[B], when the initial concentration of A is tripled keeping concentration of B constant, the initial rate would (NEET 2023)
(a) Decrease by a factor of nine
(b) Increase by a factor of six
(c) Increase by a factor of nine
(d) Increase by a factor of three
Ans: C

Q2: Given below are two statements: one is labelled as Assertion A and the other is labelled as Reason R: (NEET 2023)
Assertion (a) A reaction can have zero activation energy.
Reasons R: The minimum extra amount of energy absorbed by reactant molecules so that their energy becomes equal to threshold value, is called activation energy.
In the light of the above statements, choose the correct answer from the options given below:
(a) Both A and R are true and R is the correct explanation of A
(b) Both A and R are true and R is NOT the correct explanation of A
(c) A is true but R is false
(d) A is false but R is true
Ans: (b)
Step 1: Assertion (A)
The statement says “A reaction can have zero activation energy.” This is correct. Certain reactions, such as free-radical reactions, can proceed without any extra energy barrier.
Step 2: Reason (R)
The reason given is “The minimum extra amount of energy required by reactant molecules so that their energy becomes equal to the threshold value is called activation energy.” This is the correct definition of activation energy.
Step 3: Relationship Between A and R
Although both the assertion and the reason are true, the reason does not actually justify the assertion. Knowing the definition of activation energy does not explain why in some cases, activation energy can be zero.
Therefore, Option (b) is the correct answer.
Q3: The correct options for the rate law that corresponds to overall first order reaction is: (NEET 2023)
(a) Rate=k[A]0[B]2
(b) Rate=k[A][B]
(c) Rate=k[A]1/2[B]2
(d) Rate=k[A]−1/2[B]3/2
Ans: (d)
To understand this, let's first define what a first-order reaction means. The overall order of a reaction is the sum of the powers of the concentration terms in the rate law equation. In an overall first-order reaction, the sum of the powers should add up to 1.
Now, let's analyze the given options:
- (a) Rate = k[A]⁰[B]²: This would result in an overall order of 2, because the order of [A] is 0 and the order of [B] is 2. So, this is not a first-order reaction.
- (b) Rate = k[A][B]: This has a total order of 1 (1 for [A] and 1 for [B]), which is a second-order reaction in total, not first-order.
- (c) Rate = k[A]¹/₂[B]²: This is a mixed-order reaction with fractional exponents. The overall order is 1/2 + 2 = 2.5, which is not first order.
- (d) Rate = k[A]⁻¹/₂[B]³/₂: This gives an overall order of -1/2 + 3/2 = 1, which is first-order. Therefore, this is the correct rate law for a first-order reaction.
Thus, the correct answer is (d) Rate = k[A]⁻¹/₂[B]³/₂.
Q4: For a reaction: 3A → 2B
The average rate of appearance of B is given by Δ[B] / Δt.
The correct relation between the average rate of appearance of B and the average rate of disappearance of A is: (NEET 2023)
(a) − Δ[A] / Δt
(b) − (3 Δ[A]) / (2 Δt)
(c) − (2 Δ[A]) / (3 Δt)
(d) Δ[A] / Δt
Ans: (c)
Given the reaction:
3A → 2B
The stoichiometry of the reaction shows that for every 3 moles of A that are consumed, 2 moles of B are produced.
Now, we can write the relationships between the rates of disappearance of A and appearance of B.
- The average rate of disappearance of A is given by:
Rate of disappearance of A = −(Δ[A] / Δt) - The average rate of appearance of B is given by:
Rate of appearance of B = Δ[B] / Δt
From the stoichiometric ratio, we know that the change in the concentration of B is related to the change in the concentration of A by the ratio 2/3. This means for every 3 moles of A that disappear, 2 moles of B appear.
Therefore, the relation between the rate of disappearance of A and the rate of appearance of B is:
Δ[B] / Δt = (2/3) * (−Δ[A] / Δt)
Thus, the correct relation is: (c) − (2 Δ[A]) / (3 Δt).
2022
Q1: The given graph is a representation of kinetics of a reaction. (NEET 2022 Phase 1)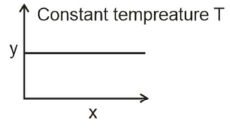 The y and x axes for zero and first order reactions, respectively are (NEET 2022)
The y and x axes for zero and first order reactions, respectively are (NEET 2022)
(a) zero order (y = rate and x = concentration), first order (y = t1/2, and x = concentration)
(b) zero order (y = rate and x = concentration), first order (y = rate and x = t1/2)
(c) zero order (y = concentration and x = time), first order (y = t1/2 and x = concentration)
(d) zero order (y = concentration and x = time), first order (y = rate constant and x = concentration)
Ans: (a)
- For zero order reaction
r = k[A]0
r = k (constant)
hence, 'y' as 'rate' and 'x' as concentration will give desired graph. - For first order reaction
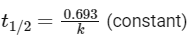
hence, 'y' as 't1/2' and 'x' as concentration will given desired graph.
Q2: For a first order reaction A → Products, initial concentration of A is 0.1 M, which becomes 0.001 M after 5 minutes. Rate constant for the reaction in min–1 is (NEET 2022 Phase 1)
(a) 0.4606
(b) 0.2303
(c) 1.3818
(d) 0.9212
Ans: (d)
A → Product
t = 0 0.1 M
t = 5 min 0.001 M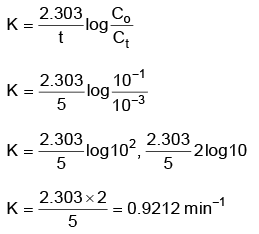
Q3: For a chemical reaction
4A + 3B
Rate of formation of C is 6
(a) 10
(b) 1
(c) 10
(d) 1
Ans: (b)
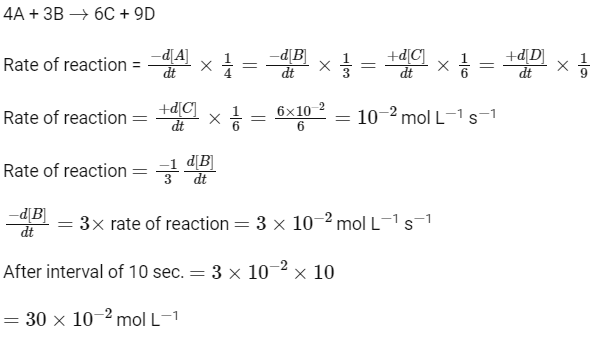
2021
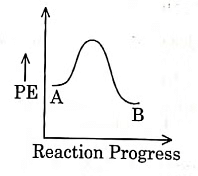
Q1: For a reaction A → B, enthalpy of reaction is –4.2 kJ mol–1 and enthalpy of activation is 9.6 kJ mol–1. The correct potential energy profile for the reaction is shown in option. (NEET 2021)
(a) 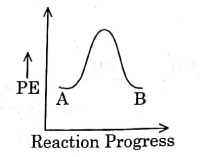
(b) 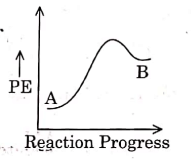
(c) 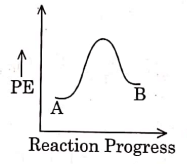
(d) 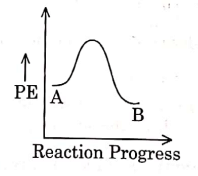
Ans: (d)
The enthalpy of reaction is negative, – 4.2 kJ mol−1 . The reaction is an exothermic reaction i.e. the energy of product B, is less than the energy of reactant A. So, the potential energy profile for the reaction is
Q2: The slope of Arrhenius Plot (ln k v/s 1/T) of the first-order reaction is –5 × 103 K. The value of Ea of the reaction is. Choose the correct option for your answer. [Given R = 8.314 JK–1 mol–1] (NEET 2021)
(a) 166 kJ mol-1
(b) -83 kJ mol-1
(c) 41.5 kJ mol-1
(d) 83.0 kJ mol-1
Ans: (c)
Arrhenius equation
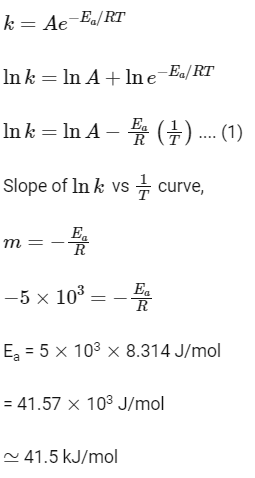
2020
Q1: An increase in the concentration of the reactants of a reaction leads to change in :
(a) heat of reaction
(b) threshold energy
(c) collision frequency
(d) activation energy (NEET 2020)
Ans: (c)
The correct answer to this question is collision frequency. Here's an explanation for each option to understand why:
- Option A - Heat of Reaction: The heat of reaction, also known as the enthalpy change (ΔH), is determined by the difference in the energy levels of the reactants and products. It is a characteristic feature of a particular chemical reaction and is not typically affected by the concentration of reactants except in cases of extremely high or non-ideal concentrations where volume changes might somewhat influence pressure and therefore the heat of reaction in gases. Under normal conditions, however, changing the concentration of reactants does not alter the heat of reaction.
- Option B - Threshold Energy: Threshold energy is the minimum energy that reactant molecules must possess in order to undergo a successful collision, leading to a chemical reaction. This value is inherent to the specific reaction and is related to the strength of the bonds in the reactants as well as the energy required to form the activated complex during the reaction. The threshold energy does not change simply because the concentration of reactants is altered.
- Option C - Collision Frequency: Collision frequency corresponds to how often reacting particles collide in a given time interval. When the concentration of reactants is increased, there are more reactant particles per unit volume. This statistically leads to an increased number of collisions per unit time, thereby raising the collision frequency. This is in accordance with the collision theory of chemical kinetics.
- Option D - Activation Energy: Activation energy is the minimum energy barrier that must be overcome for reactants to be converted into products. It is a property of a particular reaction and is related to the nature of the reactants and the reaction pathway. Altering the concentration of reactants does not change the activation energy for that reaction.
Thus, the only factor among the provided options that changes with an increase in the concentration of reactants is indeed the collision frequency.
Q2: The rate constant for a first-order reaction is 4.606 × 10-3 s-1. The time required to reduce 2.0 g of the reactant to 0.2 g is:
(a) 500 s
(b) 1000 s
(c) 100 s
(d) 200 s (NEET 2020)
Ans: (a)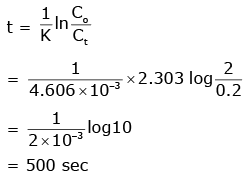
2019
Q1: If the rate constant for a first-order reaction is k, the time (t) required for the completion of 99% of the reaction is given by: (NEET 2019)
(a) t = 0.693/k
(b) t = 6.909/k
(c) t = 4.606/k
(d) t = 2.303/k
Ans: (c)
The first-order rate constant is given as,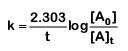
99% completed reaction,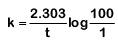
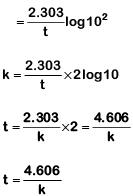
Q2: For the chemical reaction 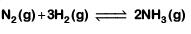 The correct option is: (NEET 2019)
The correct option is: (NEET 2019)
(a)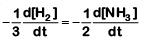
(b)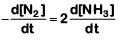
(c)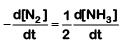
(d)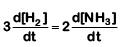
Ans: (c)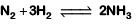
The rate of reaction is given as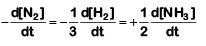
2018
Q1: The correct difference between first- and second-order reaction is that (NEET 2018)
(a) the rate of a first-order reaction does not depend on reactant concentration; the rate of a second-order reaction does depend on reactant concentrations.
(b) the half-life of a first-order reaction does not depend on [A]0; the half-life of a second-order reaction does depend on [A]0
(c) a first-order reaction can be catalyzed; a second-order reaction cannot be catalyzed.
(d) the rate of a first-order reaction does depend on reactant concentrations; the rate of a second-order reaction does not depend on reactant concentrations
Ans: (b)
For the first order reaction, t1/2 =
which is independent of initial concentration [A]0.
For second order reaction, t1/2 = 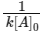
which depends on initial concentration [A]0.
Q2: When the initial concentration of the reactant is doubled, the half-life period of a zero-order reaction (NEET 2018)
(a) is halved
(b) is doubled
(c) is tripled
(d) remains unchanged
Ans: (b)
Half life of zero order
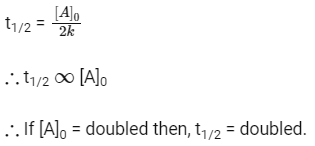
2017
Q1: Mechanism of a hypothetical reaction (NEET 2017)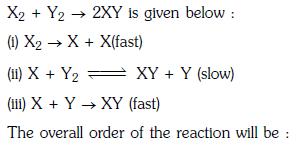
(a) 2
(b) 0
(c) 1.5
(d) 1
Ans: (c)
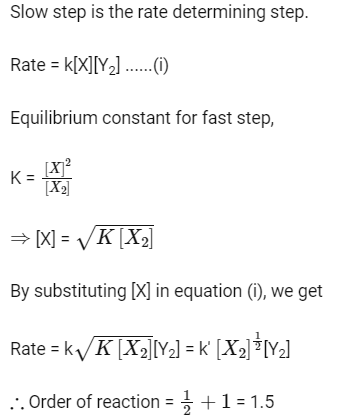
Q2: A first-order reaction has a specific reaction rate of 10–2 sec–1. How much time will it take for 20g of the reactant to reduce to 5 g ? (NEET 2017)
(a) 138.6 sec
(b) 346.5 sec
(c) 693.0 sec
(d) 238.6 sec
Ans: (a)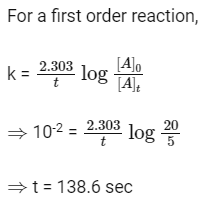
2016
Q1: The rate of a first-order reaction is 0.04 mol ℓ-1 s-1 at 10 seconds and 0.03 mol ℓ-1 s-1 at 20 seconds after initiation of the reaction. The half-life period of the reaction is : (NEET 2016 Phase 1)
(a) 54.1 s
(b) 24.4 s
(c) 34.1 s
(d) 44.1 s
Ans: (b)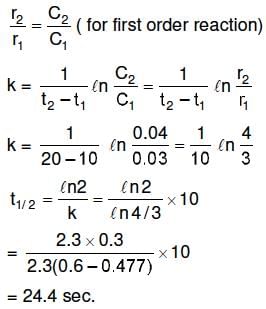
Q2: The addition of a catalyst during a chemical reaction alters which of the following quantities? (NEET 2016 Phase 1)
(a) Enthalpy
(b) Activation energy
(c) Entropy
(d) Internal energy
Ans: (b)
A catalyst provides an alternate path to the reaction which has lower activation energy.
Q3: The decomposition of phosphine (PH3) on tungsten at low pressure is a first-order reaction. It is because the
(a) rate is proportional to the surface coverage
(b) rate is inversely proportional to the surface coverage
(c) rate is independent of the surface coverage
(d) rate of decomposition is very slow. (NEET 2016 Phase 2)
Ans: (a)
At low pressure, rate is proportional to the surface coverage and is of first order while at high pressure it follows zero order kinetic due to complete coverage of surface area.
2015
Q1: The rate constant of the reaction A
(a) 3.60 M
(b) 0.36 M
(c) 0.72 M
(d) 1.08 M
Ans: (c)
For zero order reaction unit of rate constant is mole per second.
For zero order
x = kt
x = 0.6 × 10–3 × 20 × 60 = 0.72 M
Q2: The activation energy of a reaction can be determined from the slope of which of the following graphs? )
(a)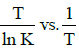
(b) ln K vs. T
(c)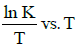
(d)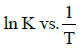 (NEET / AIPMT 2015 Cancelled Paper)
(NEET / AIPMT 2015 Cancelled Paper)
Ans: (d)
According to Arrhenius equation,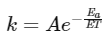
Taking natural log on both the sides we get,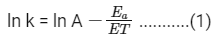
Comparing (1) with standard form of equation of line
y = mx + C
We get Slope, 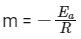
Hence, if ln k is plotted against 1/T, slope of the line will be 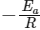 .
.
Q3: When the initial concentration of a reactant is doubled in a reaction, its half-life period is not affected. The order of the reaction is : (NEET / AIPMT 2015 Cancelled Paper)
(a) More than zero but less than first
(b) Zero
(c) First
(d) Second
Ans: (c)
Half-life period of a first order reaction is independent of initial concentration,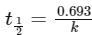
|
75 videos|278 docs|78 tests
|
FAQs on NEET Previous Year Questions (2014-2025): Chemical Kinetics - Chemistry Class 12
| 1. What is chemical kinetics and why is it important in the field of chemistry? |  |
| 2. What are the factors that can affect the rate of a chemical reaction? |  |
| 3. How can reaction mechanisms be determined in chemical kinetics? |  |
| 4. What is the difference between rate constant and rate of reaction in chemical kinetics? |  |
| 5. How can chemical kinetics be applied in real-life scenarios? |  |

















