Organic Reactions With Mechanism and Applications (Part -1) | Organic Chemistry PDF Download
⇒ Wolff rearrangement:
α-Diazoketones on treatment with solid silver oxide split off nitrogen and rearrange to ketene. This is known as Wolff rearrangement.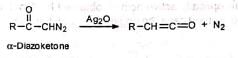 The rearrangement may also occur on irradiation or on heating. When the reaction is carried out in the presence of water, alcohol, ammonia or amine, the highly reactive ketene readily reacts with the nucleophiles present, e.g., H20, ROH. etc., to give respectively acids, esters, amides or substituted amides of the next higher homologue of the acid from which the a-diazoketone is prepared.
The rearrangement may also occur on irradiation or on heating. When the reaction is carried out in the presence of water, alcohol, ammonia or amine, the highly reactive ketene readily reacts with the nucleophiles present, e.g., H20, ROH. etc., to give respectively acids, esters, amides or substituted amides of the next higher homologue of the acid from which the a-diazoketone is prepared.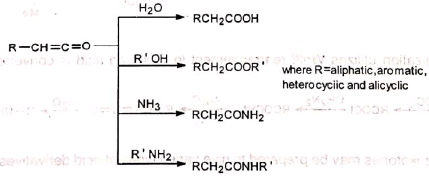
Mechanism:
It has been shown (Huggett et al.) with isotopically labelled carbon (13C) in a series of transformations that the carbonyl carbon of a-diazoketone is present in the resulting acid as the carboxyl carbon when the reaction is carried out in the presence of water. Obviously, migration must have occurred during the rearrangement. On the basis of this, the following mechanism has been suggested:
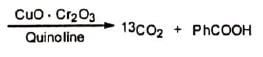
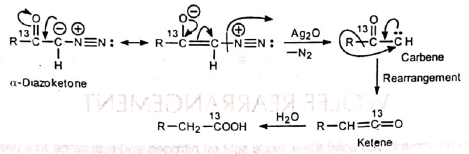 Splitting of nitrogen and migration of R group may be concerted. In some cases ketenes have been isolated. The group R migrates with retention of configuration. This has been confirmed by the following observation.
Splitting of nitrogen and migration of R group may be concerted. In some cases ketenes have been isolated. The group R migrates with retention of configuration. This has been confirmed by the following observation.
A higher homologue of an optically active acid (I) obtained by Arndt-Eistert-Wolff rearrangement on degradation by Barbier-Weiland method gave the original acid with the same configuration (Lane and Wallis).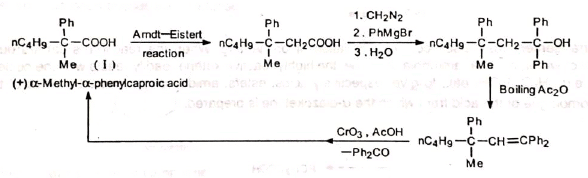
Application:
Arndt-Eistert homologization utilizes Wolff rearrangement in which an acid is converted to its next higher homologue.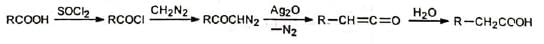 Various classes of diazoketones may be prepared to give varied types of acid derivatives for further synthetic applications.
Various classes of diazoketones may be prepared to give varied types of acid derivatives for further synthetic applications.
⇒ Darzens Condensation: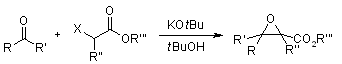 The Darzens Reaction is the condensation of a carbonyl compound with an α-halo ester in the presence of a base to form an α, β-epoxy ester.
The Darzens Reaction is the condensation of a carbonyl compound with an α-halo ester in the presence of a base to form an α, β-epoxy ester.
Mechanism of the Darzens Reaction:
After deprotonation, the α-halo ester adds to the carbonyl compound to give syn and anti diastereomers: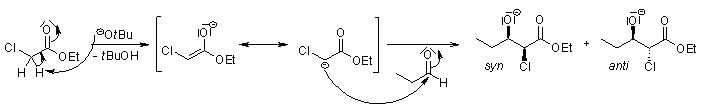 In the subsequent step, an intramolecular SN2 reaction forms the epoxide:
In the subsequent step, an intramolecular SN2 reaction forms the epoxide: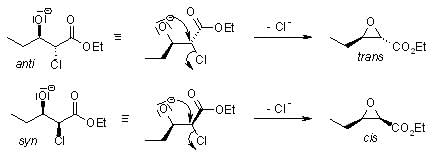 Typically, the cis:trans ratio of the epoxide formation lies between 1:1 and 1:2.
Typically, the cis:trans ratio of the epoxide formation lies between 1:1 and 1:2.
In the past, Darzens methodology was primarily used for the synthesis of aldehydes and ketones, as a homologation reaction without any consideration of stereocontrol in the epoxide formation. For this sequence, saponification of the α,β-epoxy ester followed by decarboxylation gives the substituted carbonyl compound: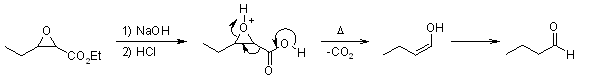
 Darzens methodology for the construction of epoxides can also be used for α-halo carbonyl compounds, or similar compounds that can undergo deprotonation and bear electron-withdrawing groups. In addition, the reaction can be carried out with diazoacetate, where N2 is the leaving group, or with a sulphur ylide with SR2 as the leaving group (see Corey Chaykovsky).
Darzens methodology for the construction of epoxides can also be used for α-halo carbonyl compounds, or similar compounds that can undergo deprotonation and bear electron-withdrawing groups. In addition, the reaction can be carried out with diazoacetate, where N2 is the leaving group, or with a sulphur ylide with SR2 as the leaving group (see Corey Chaykovsky).
In the following specific substitution pattern, the outcome of the reaction depends on the energy of the transition states of the addition, the rotation and the ring closure, as described by Aggarwal. Although explanations for the diastereoselectivity have been given, the enantioselectivity that is induced by the camphor-derived sulphonium group is not yet fully understood: Another concept for highly diastereoselective and enantioselective transformations was developed by Arai:
Another concept for highly diastereoselective and enantioselective transformations was developed by Arai: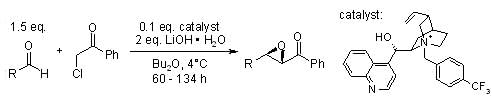 In this system, the chiral phase transfer catalyst (PTC) is able to recognize one aldolate selectively. There is an equilibrium between syn- and anti-aldolates via retro-aldol addition, and the formation of a stable, chelated lithium salt blocks the non-catalyzed subsequent reaction from yielding the epoxide product:
In this system, the chiral phase transfer catalyst (PTC) is able to recognize one aldolate selectively. There is an equilibrium between syn- and anti-aldolates via retro-aldol addition, and the formation of a stable, chelated lithium salt blocks the non-catalyzed subsequent reaction from yielding the epoxide product: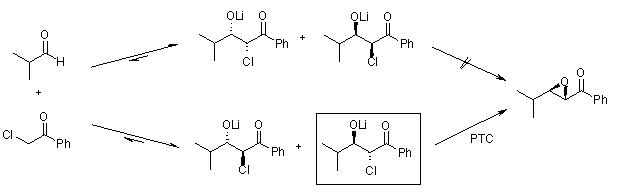 The following aza-Darzens reaction, in which a preformed lithium α-bromoenolate reacts with a sulphinimine to give an aziridine, features a six-membered transition state that accounts for the high diastereoselectivity:
The following aza-Darzens reaction, in which a preformed lithium α-bromoenolate reacts with a sulphinimine to give an aziridine, features a six-membered transition state that accounts for the high diastereoselectivity: The development of enantioselective methods remains challenging. In principle, any of the methods that are used for stereoselective aldol additions can also be tested in the Darzens Reaction, as the first step is an aldol addition.
The development of enantioselective methods remains challenging. In principle, any of the methods that are used for stereoselective aldol additions can also be tested in the Darzens Reaction, as the first step is an aldol addition.
⇒Wittig reaction:
Wittig reaction affords an important and useful method for the synthesis of alkenes by the treatment of aldehydes or ketones with alkylidenetriphenylphosphorane (Ph3P=CR2) or simply known as phosphorane.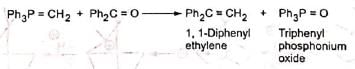 The Wittig reagent, alkylidenetriphenylphosphorane, is prepared by treating trialkyl or triarylphosphine usually the latter with an alkyl halide in ether solution. The resulting phosphonium salt is treated with a strong base (such as C6H5Li, BuLi, NaNH2, NaH, C2H5ONa, etc.) which removes a halacid to give the reagent, methylenetriphenyl phosphorane (II).
The Wittig reagent, alkylidenetriphenylphosphorane, is prepared by treating trialkyl or triarylphosphine usually the latter with an alkyl halide in ether solution. The resulting phosphonium salt is treated with a strong base (such as C6H5Li, BuLi, NaNH2, NaH, C2H5ONa, etc.) which removes a halacid to give the reagent, methylenetriphenyl phosphorane (II).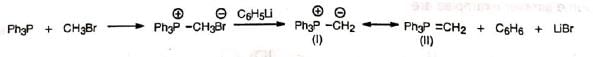 In the alkyl halide a hydrogen is necessary on the halogen-bearing carbon. Alkylidenetriphenylphosphoranes are also called ylids due to the presence of opposite formal charges on adjacent atoms as in one of the resonance structures (I). The methylene structure (II) has a dπ-pπ bond between phosphorus and carbon. The ylid may be considered as a carbanion stabilized by the adjacent phosphonium cation.
In the alkyl halide a hydrogen is necessary on the halogen-bearing carbon. Alkylidenetriphenylphosphoranes are also called ylids due to the presence of opposite formal charges on adjacent atoms as in one of the resonance structures (I). The methylene structure (II) has a dπ-pπ bond between phosphorus and carbon. The ylid may be considered as a carbanion stabilized by the adjacent phosphonium cation.
The carbonyl compound is directly treated with the ethereal solution of the reagent.
Mechanism:
The reaction probably proceeds by the nucleophilic attack of the ylid on the carbonyl carbon. The dipolar complex (betain) so formed decomposes to olefine and triphenylphosphine oxide via a four-centred transition state.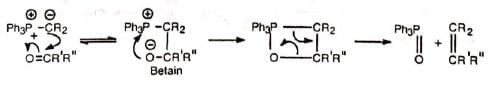 The mechanism is supported by the fact that an optically active phosphonium salt reacts to produce a phosphine oxide with retention of configuration.
The mechanism is supported by the fact that an optically active phosphonium salt reacts to produce a phosphine oxide with retention of configuration.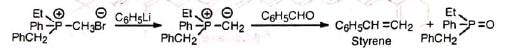 Since desired alkyl groups can be introduced in the alkyl halide and the carbonyl compound, it is extremely useful for the synthesis of desired substituted alkenes. Double or triple bonds even when conjugated with the carbonyl group
Since desired alkyl groups can be introduced in the alkyl halide and the carbonyl compound, it is extremely useful for the synthesis of desired substituted alkenes. Double or triple bonds even when conjugated with the carbonyl group 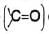 does not interfere. The reaction with the carbonyl group of esters is very slow and does not interfere.
does not interfere. The reaction with the carbonyl group of esters is very slow and does not interfere.
Phosphorous ylids react in the same manner with the C=O groups of ketenes and isocyanates as also with the N=O and C=N groups of nitroso and imine compounds respectively.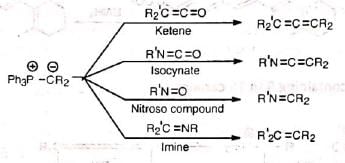
Applications:
The reaction has many useful synthetic applications. Many natural products which are otherwise difficult to prepare can be synthesized by Wittig reaction.
1. Formation of exocyclic methylene group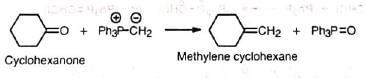 This method of introducing exocyclic methylene group is extremely valuable and has been widely used in the preparation of methylene steroids.
This method of introducing exocyclic methylene group is extremely valuable and has been widely used in the preparation of methylene steroids.
2. Preparation of β, γ-unsaturated acids In all other methods isomerization to α, β-unsaturated acids results.
In all other methods isomerization to α, β-unsaturated acids results.
3. Preparation of natural products
(i) β-carotene (pro vitamin A)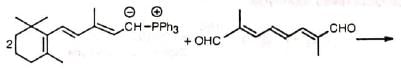
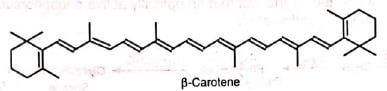 (ii) Vitamin A
(ii) Vitamin A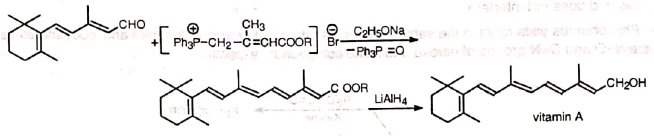 4. Formation of large rings containing 5 to 16 carbons
4. Formation of large rings containing 5 to 16 carbons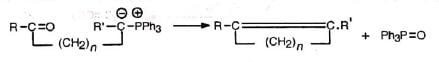 5. Synthesis of vinyl halides Chloromethylenetriphenylphosphorane (Ph3P=CHCI) required for this synthesis is prepared by reacting chlorocarbene with triphenylphosphine.
5. Synthesis of vinyl halides Chloromethylenetriphenylphosphorane (Ph3P=CHCI) required for this synthesis is prepared by reacting chlorocarbene with triphenylphosphine.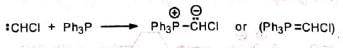
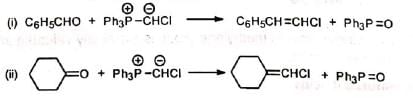 6. Synthesis of ethers Methoxymethylenetriphenylphosphorane reacts with carbonyl compounds to give diphenyl substituted vinyl methyl ethers.
6. Synthesis of ethers Methoxymethylenetriphenylphosphorane reacts with carbonyl compounds to give diphenyl substituted vinyl methyl ethers.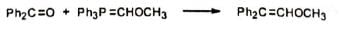 Hydrolysis of the product gives aldehyde.
Hydrolysis of the product gives aldehyde.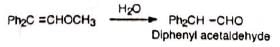
⇒ Michael Reaction:
The base-catalysed addition of compounds having active methylene group (or relatively acidic hydrogens) to an activated olefinic bond of the type 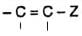 ( Z = electron-withdrawing) is classified as Michael reaction.
( Z = electron-withdrawing) is classified as Michael reaction.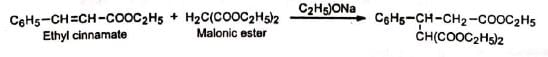 The compounds having an active methylene group or having relatively acidic hydrogens are called donors and the compounds having an activated olefinic bond are called acceptors. A large variety of donors and acceptors are employed in Michael reaction.
The compounds having an active methylene group or having relatively acidic hydrogens are called donors and the compounds having an activated olefinic bond are called acceptors. A large variety of donors and acceptors are employed in Michael reaction.
The donors include malonic ester, cyanoacetic ester, acetoacetic ester, phenylacetic acid esfer, cyanoacetamide, aliphatic nitro compounds, benzyl cyanide, sulphones, cyclopentadienes, indenes, fluorenes, etc.
The acceptors include
(a) aldehydes, e.g., a craldehyde, CH2 = CH-CHO; cinnamaldehyde, C6H5CH = CHCHO.
(b) ketones, e.g., benzylideneacetone, C6H5CH = CHCOCH3; mesityloxide, (CH3)2C = CHCOCH3; quinones, etc.
(c) nitriles, e.g., acrylonitrile, CH2=CH-CN.
(d) esters of a, p-unsaturated acids, e.g., C6H5-CH=CH-COOC2H5.
Various types of basic catalysts are used. Most commonly used are alkali metal alkoxides, such as sodium or potassium ethoxides, potassium tertiary butoxide, potassium isopropoxide, etc. Mild basic catalysts such as 2° amines, 3° amines, piperidine and pyridine have been used with success.
Mechanism:
The base generates a carbanion from the donor, malonic ester. The carbanion then adds to the β-carbon of the α, β-unsaturated ester acceptor, ethyl cinnamate to yield the anion (I) which takes up a proton from alcohol to produce an enol. The enol then tautomerises to the more stable product, ketone.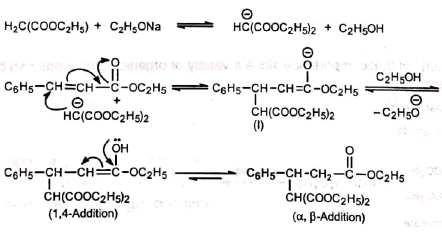 The electron-attracting group -COOC2H5 (Z) facilitates the attack by stabilizing the intermediate anion (I) by dispersal of the charge. It is seen that although 1, 4-addition occurs initially, the final result is addition to the α, β-unsaturated carbons. This is because the enol reverts to the more stable ketone (recall that vinyl alcohol is unknown). In the presence of strong base, the product may undergo cyclisation. No cyclisation occurs with mild bases such as 2° or 3° amines and piperidine.
The electron-attracting group -COOC2H5 (Z) facilitates the attack by stabilizing the intermediate anion (I) by dispersal of the charge. It is seen that although 1, 4-addition occurs initially, the final result is addition to the α, β-unsaturated carbons. This is because the enol reverts to the more stable ketone (recall that vinyl alcohol is unknown). In the presence of strong base, the product may undergo cyclisation. No cyclisation occurs with mild bases such as 2° or 3° amines and piperidine.
Compounds with two double bonds in conjugation with the electron-withdrawing group may undergo nucleophilic attack at β-carbon or δ-carbon to give three products. Thus:
(a) On attack at β-carbon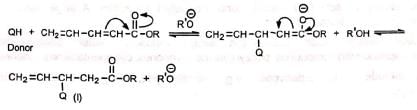 (b) On attack at δ-carbon
(b) On attack at δ-carbon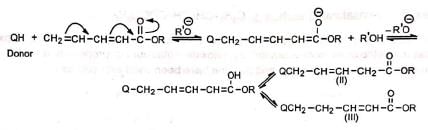 Since the double bond is conjugated in (III), it is the most stable of the three products. Hence it is the predominant product.
Since the double bond is conjugated in (III), it is the most stable of the three products. Hence it is the predominant product.
Applications:
The reaction is of great synthetic importance since a variety of organic compounds can be synthesized with the help of this reaction.
1. Synthesis of polybasic acids
(a) Tricarballylic acids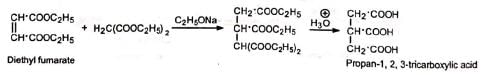 Esters of tricarballylic acid are used as plasticisers,
Esters of tricarballylic acid are used as plasticisers,
(b) Aconitic acid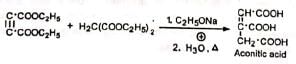 Aconitic acid is used in the preparation of medicines for releaving pain (analgesic) and reducinq fever (febrifuge). Its esters are used as plasticisers.
Aconitic acid is used in the preparation of medicines for releaving pain (analgesic) and reducinq fever (febrifuge). Its esters are used as plasticisers.
2. Preparation of cyano and nitro compounds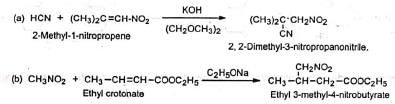 3. Building of ring system
3. Building of ring system
(a) Condensed alicyclic ring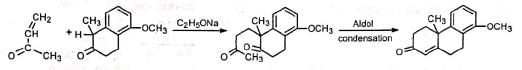 This method has been employed during the synthesis of cholesterol to build the ring system,
This method has been employed during the synthesis of cholesterol to build the ring system,
(b) Double Michael addition for ring formation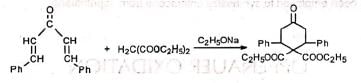 (c) Cyclopropane derivative—Caronic acid
(c) Cyclopropane derivative—Caronic acid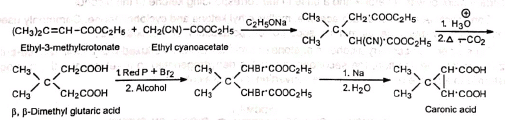 4. Synthesis of dimedone Dimedone is a reagent for the identification of aldehydes in the presence of ketones.
4. Synthesis of dimedone Dimedone is a reagent for the identification of aldehydes in the presence of ketones.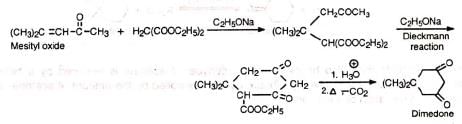 Dimedone is also employed for the separation of aldehydes from ketones because it reacts only with aldehydes.
Dimedone is also employed for the separation of aldehydes from ketones because it reacts only with aldehydes.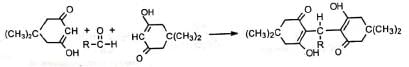 Reaction with formaldehyde is quantitative and hence, the reagent is used for the quantitative estimation of formaldehyde.
Reaction with formaldehyde is quantitative and hence, the reagent is used for the quantitative estimation of formaldehyde.
5. Synthesis of amino acids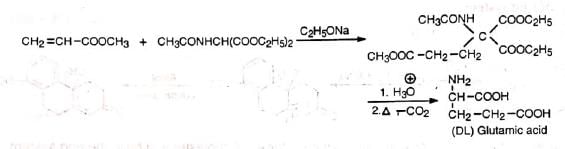 Michael reaction has been employed to synthesize anthracene from naphthalene.
Michael reaction has been employed to synthesize anthracene from naphthalene.
|
35 videos|92 docs|46 tests
|
FAQs on Organic Reactions With Mechanism and Applications (Part -1) - Organic Chemistry
| 1. What is the definition of organic reactions? |  |
| 2. What is the importance of understanding the mechanism of organic reactions? |  |
| 3. Are all organic reactions accompanied by a detailed mechanism? |  |
| 4. What are some common applications of organic reactions? |  |
| 5. How can knowledge of organic reactions be applied in everyday life? |  |

|
Explore Courses for Chemistry exam
|

|

















