NEET Previous Year Questions (2014-2024): Nuclei | Physics Class 12 PDF Download
2024
Q1: 
In the nuclear emission stated above, the mass number and atomic number of the product Q respectively, are
(a) 280, 81
(b) 286, 80
(c) 288, 82
(d) 286, 81 [2024]
Ans: (d)
To determine the mass number and atomic number of the final product Q in the provided nuclear reactions, we need to analyze the impact of each type of decay (alpha, positron emission, beta-minus decay, and electron capture) on the mass number and atomic number of the initial element 
1. Alpha decay (α decay): In alpha decay, an alpha particle (which is a  nucleus) is emitted. This reduces the mass number by 4 units and the atomic number by 2 units.
nucleus) is emitted. This reduces the mass number by 4 units and the atomic number by 2 units.
2. Beta plus decay (positron emission, e+): During positron emission, a proton in the nucleus is transformed into a neutron, and a positron is emitted. This decreases the atomic number by 1 but does not change the mass number.
3. Beta-minus decay (β− decay): In a beta-minus decay, a neutron in the nucleus converts into a proton and an electron (beta particle) and an antineutrino are emitted. This results in an increase in the atomic number by 1, while the mass number remains unchanged.
4. Electron capture (e− capture): During electron capture, an atomic electron is absorbed by the nucleus, causing a proton to convert into a neutron. This process decreases the atomic number by 1 without altering the mass number.
From the calculations above, the mass number of Q is 286, and its atomic number is 79. Comparing these values with the multiple choices:
Option A: Mass number = 280, Atomic number = 81
Option B: Mass number = 286, Atomic number = 80
Option C: Mass number = 288, Atomic number = 82
Option D: Mass number = 286, Atomic number = 81
None of these match exactly, implying an error in the problem or the answer options. Based on the correct atomic number calculations and assuming option D is meant to be atomic number 79, then the corrected choice would have been:
D: Mass number = 286, Atomic number = 79
2023
Q.1. The half life of a radioactive substance is 20 minutes. In how much time, the activity of substance drops to (1/16)th of its initial value? (2023)
A: 20 minutes
B: 40 minutes
C: 60 minutes
D: 80 minutes
Ans: d
Solution: 
2022
Q.2. In the given nuclear reaction, the element X is (2022)
A: 
B: 
C: 
D: 
Ans: B
Solution: 
2021
Q.3. A nucleus with mass number 240 breaks into fragments each of mass number 120, the binding energy per nucleon of unfragmented nuclei is 7.6 MeV while that of fragments is 8.5 MeV. The total gain in the Binding Energy in the process is: (2021)
A: 804 MeV
B: 216 MeV
C: 0.9 MeV
D: 9.4 MeV
Ans: B
Solution:
Given binding energy per nucleon of X, Y & Z are 7.6 MeV, 8.5 MeV & 8.5 MeV respectively.
Gain in binding energy is :-
Q = Binding Energy of products – Binding energy of reactants
= (120 × 8.5 × 2) – (240 × 7.6) MeV
= 216 MeV
Q.4. A radioactive nucleus 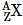 undergoes spontaneous decay in the sequence
undergoes spontaneous decay in the sequence
Where Z is the atomic number of element X. The possible decay particles in the sequence are : (2021)
A: β+,α,β-
B: β-,α,β+
C: α,β-,β+
D: α,β+,β-
Ans: A
Solution: β+ decreases atomic number by 1
β+ decreases atomic number by 1
α decreases atomic number by 2
β- decreases atomic number by 1
Q.5. The half-life of a radioactive nuclide is 100 hours. The fraction of original activity that will remain after 150 hours would be: (2021)
A: 2/3
B: 2/3√2
C: 1/2
D: 1/2√2
Ans: D
Solution: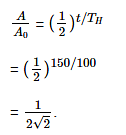
2020
Q.6. The energy equivalent of 0.5 g of a substance is : (2020)
A: 1.5×1013 J
B: 0.5×1013J
C: 4.5×1016J
D: 4.5×1013J
Ans: D
Solution:
E = Δmc2 = 0.5 × 10-3 × (3 × 108)2
= 0.5 ×10-3 × 9 ×1016
E = 4.5 ×1013 J
Q.7. When a uranium isotope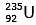 is bombarded with a neutron, it generates
is bombarded with a neutron, it generates 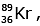 three neutrons and : (2020)
three neutrons and : (2020)
A: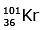
B: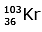
C: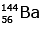
D: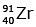
Ans: C
Solution: 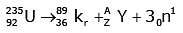
92 = 36 + Z; ← A + 89 + 3 = 235 ⇒ A = 144
Z = 56
2019
Q.8. For a radioactive material, half-life is 10 minutes. If initially there are 600 number of nuclei, the time taken (in minutes) for the disintegration of 450 nuclei is: (2018)
A: 20
B: 10
C: 30
D: 15
Ans: A
Solution: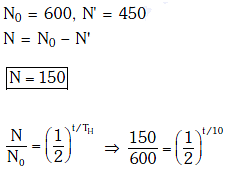

2017
Q.9. Radioactive material 'A' has decay constant '8 λ' and material 'B' has decay constant 'λ'. Initially they have same number of nuclei. After what time, the ratio of number of nuclei of material 'B' to that 'A' will be 1/e? (2017)
A: 1/7λ
B: 1/8λ
C: 1/9λ
D: 1/10λ
Ans: A
Solution: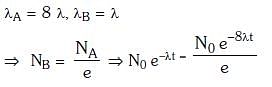
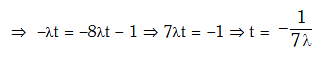

2014
Q.10. The Binding energy per nucleon of 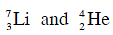 nuclei are 5.60 MeV and 7.06 MeV, respectively. In the nuclear reaction
nuclei are 5.60 MeV and 7.06 MeV, respectively. In the nuclear reaction 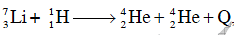 the value of energy Q released is : (2014)
the value of energy Q released is : (2014)
A: 8.4 MeV
B: 17.3 MeV
C: 19.6 MeV
D: −2.4 MeV
Ans: (b)
Solution: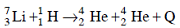
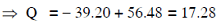
Q.11. A radio isotope ‘X’ with a half life 1.4 × 109 years decays to ‘Y’ which is stable. A sample of the rock from a cave was found to contain ‘X’ and ‘Y’ in the ratio 1 : 7. The age of the rock is (2014)
A: 4.20 × 109 years
B: 8.40 × 109 years
C: 1.96 × 109 years
D: 3.92 × 109 years
Ans: A
Solution:
Ratio of X:Y is given = 1:7
That is,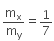
⇒ 7mx = my
Let, the initial total mass is m.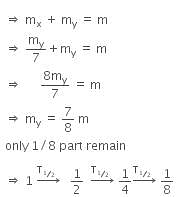
Therefore, time taken to become 1/8 unstable part
= 3 x T1/2
= 3 x 1.4 x 109
= 4.2 x 109 y
|
97 videos|336 docs|104 tests
|
FAQs on NEET Previous Year Questions (2014-2024): Nuclei - Physics Class 12
| 1. What is the nucleus in an atom? |  |
| 2. What is the significance of the nucleus in an atom? |  |
| 3. How does the nucleus contribute to the mass of an atom? |  |
| 4. What are isotopes in relation to the nucleus of an atom? |  |
| 5. How does the nucleus of an atom undergo radioactive decay? |  |

|
Explore Courses for NEET exam
|

|


















