NCERT Exemplar: Amines | Chemistry Class 12 - NEET PDF Download
| Table of contents |

|
| Multiple Choice Questions -I |

|
| Multiple Choice Questions -II |

|
| Short Answer Type Questions |

|
| Matching Type |

|
| Assertion and Reason Type Questions |

|
| Long Answer Type Questions |

|
Multiple Choice Questions -I
Q.1. Which of the following is a 3° amine?
(1) 1-methylcyclohexylamine
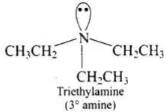
(2) Triethylamine
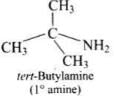
(3) Tert-butylamine
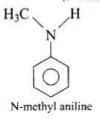
(4) N-methylaniline
Ans. (2)
Solution.
(1)
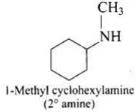
(2)
(3)
(4)
Q.2. The correct IUPAC name for CH2=CHCH2NHCH3 is
(1) Allylmethylamine
(2) 2-amino-4-pentene
(3) 4-aminopent-1-ene
(4) N-methylprop-2-en-1-amine
Ans. (4)
Solution.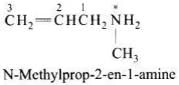
Q.3. Amongst the following, the strongest base in aqueous medium is _________.
(1) CH3NH2
(2) NCCH2NH2
(3) (CH3)2 NH
(4) C6H5NHCH3
Ans. (3)
Solution.
2° amine is more basic than 1° amine, i.e., (CH3)2NH is more basic than CH3NH2. Due to -I effect of CN group, NC-CH2NH2 is less basic than CH3NH2. Further C6H5NHCH3 is less basic than both CH3NH2 and (CH3)2NH due to delocalization of lone pair of electrons present on the nitrogen atom into benzene ring. Hence, the decreasing order of amines is:
(CH3)2NH > CH3NH2 > C6H5NHCH3 > NC – CH2NH2
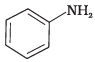
Q.4. Which of the following is the weakest Brönsted base?
(1) 
(2)
(3)
(4) CH3NH2
Ans. (1)
Solution.
Due to delocalization of lone pair of electrons on the N-atom into the benzene ring, C6H5NH2 is the weakest base.
Resonating Structure of Aniline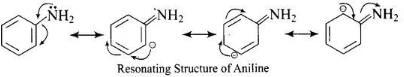
Q.5. Benzylamine may be alkylated as shown in the following equation :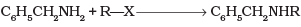
Which of the following alkylhalides is best suited for this reaction through SN1 mechanism?
(1) CH3Br
(2) C6H5Br
(3) C6H5CH2Br
(4) C2H5Br
Ans. (3)
Solution.
SN1 reaction occurs in two steps. In first step R – X bond is broken to produce a carbocation which is attacked by nucleophile. The greater the stability of carbocation, the greater will be the rate of reaction. Benzylic halides show high reactivity towards SN1 reaction.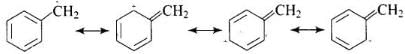
Q.6. Which of the following reagents would not be a good choice for reducing an aryl nitro compound to an amine?
(1) H2 (excess)/Pt
(2) LiAlH4 in ether
(3) Fe and HCl
(4) Sn and HCl
Ans. (2)
Solution.
Aryl nitro compound cannot be converted into amine using LiAlH4 in ether.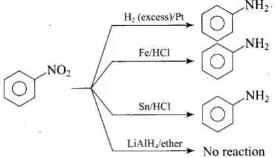
Q.7. In order to prepare a 1° amine from an alkyl halide with simultaneous addition of one CH2 group in the carbon chain, the reagent used as source of nitrogen is ___________.
(1) Sodium amide, NaNH2
(2) Sodium azide, NaN3
(3) Potassium cyanide, KCN
(4) Potassium phthalimide, C6H4(CO)2NK+
Ans. (3)
Solution.
KCN is used to increase number of carbon atoms.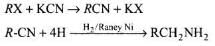
Q.8. The source of nitrogen in Gabriel synthesis of amines is ___________.
(1) Sodium azide, NaN3
(2) Sodium nitrite, NaNO2
(3) Potassium cyanide, KCN
(4) Potassium phthalimide, C6H4(CO)2N– K+
Ans. (4)
Solution.
Potassium phthalimide is the source of nitrogen in Gabriel’s synthesis.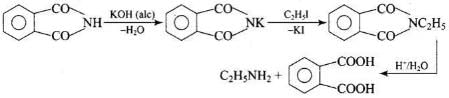
Q.9. Amongst the given set of reactants, the most appropriate for preparing 2° amine is _____.
(1) 2° R — Br + NH3
(2) 2° R — Br + NaCN followed by H2/Pt
(3) 1° R — NH2 + RCHO followed by H2/Pt
(4) 1° R — Br (2 mol) + potassium phthalimide followed by H3O+/heat
Ans. (3)
Solution.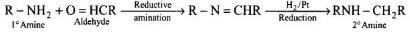
Q.10. The best reagent for converting 2–phenylpropanamide into 2-phenylpropanamine is _____.
(1) Excess H2
(2) Br2 in aqueous NaOH
(3) Iodine in the presence of red phosphorus
(4) LiAlH4 in ether
Ans. (4)
Solution.
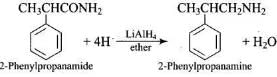
Q.11. The best reagent for converting, 2-phenylpro-panamide into 1- phenylethana-mine is ____.
(1) Excess H2/Pt
(2) NaOH/Br2
(3) NaBH4/methanol
(4) LiAlH4/ether
Ans. (2)
Solution.
Q.12. Hoffmann Bromamide Degradation reaction is shown by __________.
(1) ArNH2
(2) ArCONH2
(3) ArNO2
(4) ArCH2NH2
Ans. (2)
Solution.
Hofmann bromamidc degradation is shown by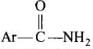 by which amide is converted into amine via undergoing intramolecular migration of phenyl group.
by which amide is converted into amine via undergoing intramolecular migration of phenyl group.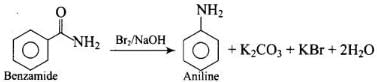
Q.13. The correct increasing order of basic strength for the following compounds is _________.
(I)
(II)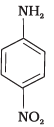
(III)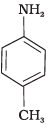
(1) II < III < I
(2) III < I < II
(3) III < II < I
(4) II < I < III
Ans. (4)
Solution.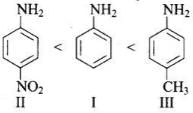
Electron withdrawing group decreases the basic strength while electron releasing groups increases the basic strength of aniline.
Q.14. Methylamine reacts with HNO2 to form _________.
(1) CH3—O—N = O
(2) CH3—O—CH3
(3) CH3OH
(4) CH3CHO
Ans. (3)
Solution.
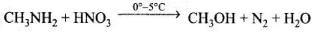
Q.15. The gas evolved when methylamine reacts with nitrous acid is __________.
(1) NH3
(2) N2
(3) H2
(4) C2H6
Ans. (2)
Solution.
Chemical reaction takes place during reaction of methylamine with nitrous acid is as follows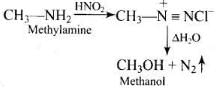
Q.16. In the nitration of benzene using a mixture of conc. H2SO4 and conc. HNO3, the species which initiates the reaction is __________.
(1) NO2
(2) NO+
(3) NO2+
(4) NO2–
Ans. (3)
Solution.
NO2 (Nitronium ion) electrophile initiates the process of nitration. It is obtained as: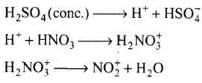
Q.17. Reduction of aromatic nitro compounds using Fe and HCl gives __________.
(1) Aromatic oxime
(2) Aromatic hydrocarbon
(3) Aromatic primary amine
(4) Aromatic amide
Ans. (3)
Solution.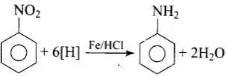
Q.18. The most reactive amine towards dilute hydrochloric acid is ___________.
(1) CH3—NH2
(2)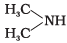
(3)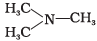
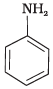
(4)
Ans. (2)
Solution.
The greater will be the strength of base, the greater will be its reactivity towards dilute HCl. Hence, (CH3)2NH has the highest basic strength as it has the highest reactivity.
Q.19. Acid anhydrides on reaction with primary amines give ____________.
(1) Amide
(2) Imide
(3) Secondary amine
(4) Imine
Ans. (1)
Solution.
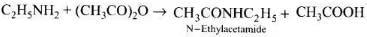
Q.20. The reaction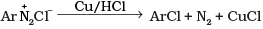 is named as _________.
is named as _________.
(1) Sandmeyer reaction
(2) Gatterman reaction
(3) Claisen reaction
(4) Carbylamine reaction
Ans. (2)
Solution.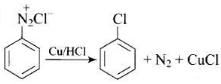
is named Gatterman reaction.
Q.21. Best method for preparing primary amines from alkyl halides without changing the number of carbon atoms in the chain is
(1) Hoffmann Bromamide reaction
(2) Gabriel phthalimide synthesis
(3) Sandmeyer reaction
(4) Reaction with NH3
Ans. (2)
Solution.
Gabriel phthalimide synthesis is used to get primary amine from alkyl halide without any change in number of carbon atoms.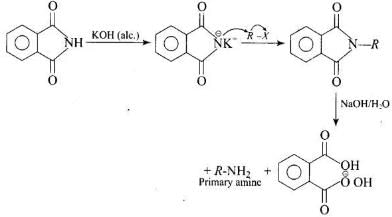
Q.22. Which of the following compound will not undergo azo coupling reaction with benzene diazonium chloride.
(1) Aniline
(2) Phenol
(3) Anisole
(4) Nitrobenzene
Ans. (4)
Solution.
Diazonium cation is a weak electrophile and hence reacts with electron rich compounds containing electron donating groups such as -OH, -NH2 and -OCH3 groups and not with compounds containing electron withdrawing groups such as -NO2, etc.
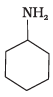
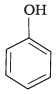
Q.23. Which of the following compounds is the weakest Brönsted base?
(1)
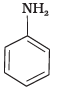
(2)
(3)
(4)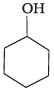
Ans. (3)
Solution.
Amines (1, 2) have stronger tendency to accept a proton and hence are stronger Bronsted bases than phenol (3) and alcohol (4). Since phenol is more acidic than alcohol, therefore, phenol (3) has the least tendency to accept a proton and hence it is the weakest Bronsted base.
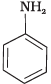
Q.24. Among the following amines, the strongest Brönsted base is __________.
(1)
(2) NH3
(3)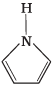
(4)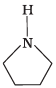
Ans. (4)
Solution.
Aniline is weaker base than NH3 due to delocalization of lone pair of electrons on the N-atom into the benzene ring. Pyrrole (3) is not at all basic because the lone pair of electrons on N-atom is donated towards aromatic sextet formation. Therefore, pyrrolidine (4) has a strong tendency to accept a proton and is hence, the strongest base.
Q.25. The correct decreasing order of basic strength of the following species is _______.
H2O, NH3, OH–, NH2–
(1) NH2– > OH- > NH3 > H2O
(2) OH– > NH2– > H2O > NH3
(3) NH3 > H2O > NH2– > OH–
(4) H2O > NH3 > OH– > NH2–
Ans. (1)
Solution.
NH2 > OH > NH3 > H2O. Due to higher electronegativity of O than N atom, O – H bond is more polar than N – H bond. Hence, O – H is more acidic in nature than N – H bond. Now, NH2 and OH have negative charge due to which they are more basic than NH3 and H2O.
Q.26. Which of the following should be most volatile?
(I) CH3CH2CH2NH2
(II) (CH3)3N
(III)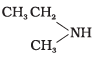
(IV) CH3CH2CH3
(1) II
(2) IV
(3) I
(4) III
Ans. (2)
Solution.
1° and 2° amines have higher boiling points due to intermolecular H-bonding (and hence less volatile than 3° amines and hydrocarbons of comparable molecular mass).
Q.27. Which of the following methods of preparation of amines will give same number of carbon atoms in the chain of amines as in the reactant?
(1) Reaction of nitrite with LiAlH4.
(2) Reaction of amide with LiAlH4 followed by treatment with water.
(3) Heating alkylhalide with potassium salt of phthalimide followed by hydrolysis.
(4) Treatment of amide with bromine in aqueous solution of sodium hydroxide.
Ans. (4)
Solution.
Aliphatic and arylalkyl primary amines can be prepared by the reduction of the corresponding nitriles with LiAlH4.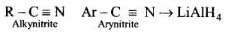
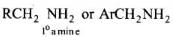
Heating alkyl halide with primary, secondary and tertiary amine can be prepared by reduction of LiAlH4 followed by treatment with water.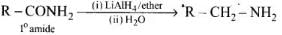
Heating alkyl halide with potassium salt of phthalimide followed by hydrolysis produces primary amine. This process is known as Gabriel phthalimide reaction. The number of carbon atoms in the chain of amines of product is same as reactant.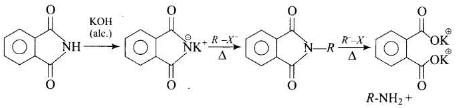
Multiple Choice Questions -II
Note : In the following questions two or more options may be correct.
Q.28. Which of the following cannot be prepared by Sandmeyer’s reaction?
(1) Chlorobenzene
(2) Bromobenzene
(3) Iodobenzene
(4) Fluorobenzene
Ans. (3, 4)
Solution.
Chloro and bromo arenes are easily prepared by Sandmeyer’s reaction. Iodoarenes are prepared by simply warming the diazonium salt solution with aqueous KI solution. Fluoroarenes are prepared by Balz-Schiemann reaction. All other reagents give aniline.
Q.29. Reduction of nitrobenzene by which of the following reagent gives aniline?
(1) Sn/HCl
(2) Fe/HCl
(3) H2-Pd
(4) Sn/NH4OH
Ans. (1, 2, 3)
Solution.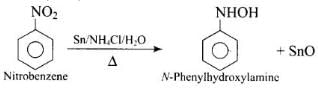
Q.30. Which of the following species are involved in the carbylamine test?
(1) R—NC
(2) CHCl3
(3) COCl2
(4) NaNO2 + HCl
Ans. (1, 2)
Solution.
Carbylamine reaction: Amine on reaction with a mixture of CHCl3 and KOH produces alkyl isocyanate.
R-NH2 + CHCl, + 3KOH → RNC + 3KCl + 3H2O Only RNC and CHCl3 are involved in carbylamine reaction.
Q.31. The reagents that can be used to convert benzenediazonium chloride to benzene are __________.
(1) SnCl2/HCl
(2) CH3CH2OH
(3) H3PO2
(4) LiAlH4
Ans. (1, 2)
Solution.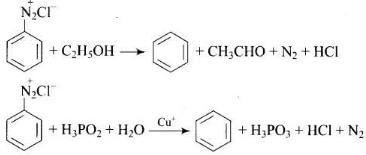
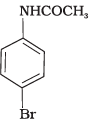
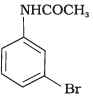
Q.32. The product of the following reaction is __________.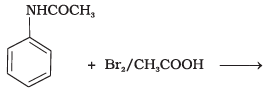 (1)
(1)
(2)
(3)
(4)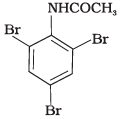
Ans. (1, 2)
Solution.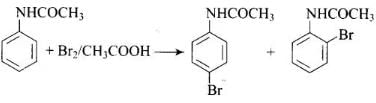
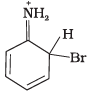
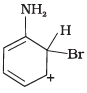
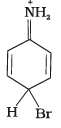
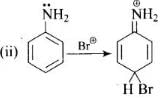
Q.33. Arenium ion involved in the bromination of aniline is __________.
(1)
(2)
(3)
(4)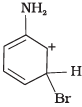
Ans. (1, 2, 3)
Solution.
Arenium ion involved in the bromination of aniline are as follows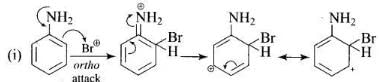
Q.34. Which of the following amines can be prepared by Gabriel synthesis.
(1) Isobutyl amine
(2) 2-Phenylethylamine
(3) N-methylbenzylamine
(4) Aniline
Ans. (1, 2)
Solution.
Only primary aliphatic amines such as
(1) (CH3)2CH-CH2NH2 and C6H5CH2NH2
(2) can be prepared by Gabriel synthesis. 2° amines, i.e., C6H5CH2NHCH3
(3) and 1° amine, C6H5NH2
(4), however, cannot be prepared.
Q.35. Which of the following reactions are correct?
(1)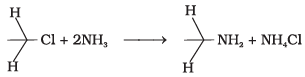
(2) 
(3)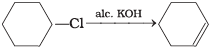
(4)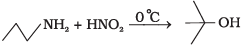
Ans. (3)
Solution.
CH3CH2NH2 + NH4Cl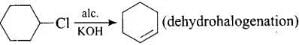
Q.36. Under which of the following reaction conditions, aniline gives p-nitro derivative as the major product?
(1) Acetyl chloride/pyridine followed by reaction with conc. H2SO4 + conc. HNO3.
(2) Acetic anyhdride/pyridine followed by conc. H2SO4 + conc. HNO3.
(3) Dil. HCl followed by reaction with conc. H2SO4 + conc. HNO3.
(4) Reaction with conc. HNO3 + conc.H2SO4.
Ans. (1, 2)
Solution.
Aniline or reaction with acetyl chloride or acetic anhydride in the presence of pyridine produces N-acetyl aniline which is a ortho, para directing group which on further reaction with nitrating mixture (cone. HNO3 + cone. H2SO4) produces p-nitroaniline preferentially as shown below: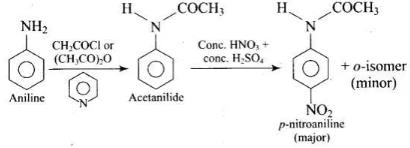
Q.37. Which of the following reactions belong to electrophilic aromatic substitution?
(1) Bromination of acetanilide
(2) Coupling reaction of aryldiazonium salts
(3) Diazotisation of aniline
(4) Acylation of aniline
Ans. (1, 2)
Solution.
Acylation is a nucleophilic substitution reaction in which H atom of -NH2 is replaced by acyl group. Diazotisation is also a nucleophilic substitution reaction.
Short Answer Type Questions
Q.38. What is the role of HNO3 in the nitrating mixture used for nitration of benzene?
Ans. HNO3 acts as a base in the nitrating mixture (HNO3 + H2SO4) and provides the electrophile.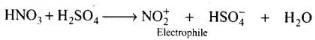
Q.39. Why is NH2 group of aniline acetylated before carrying out nitration?
OR
Why does acetylation of —NH2 group of aniline reduce its activating effect?
Ans. Direct nitration of aniline is not possible on account of oxidation of -NH2 group. However, nitration can be carried after protecting the -NH2 group by acetylation to give acetanilide which is then nitrated and finally hydrolysed to give o- and p-nitroanilines.
The acetyl group being electron withdrawing attracts the lone pair of electrons of the N-atom towards carbonyl group.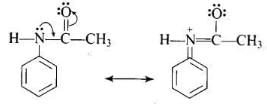 As a result, the activating effect -NH2 group is reduced i.e., the lone pair of electrons on nitrogen is less available for donation to benzene ring by resonance. Therefore, activating effect of —NHCOCH3 group is less than that of —NH2 group.
As a result, the activating effect -NH2 group is reduced i.e., the lone pair of electrons on nitrogen is less available for donation to benzene ring by resonance. Therefore, activating effect of —NHCOCH3 group is less than that of —NH2 group.
Q.40. What is the product when C6H5CH2NH2 reacts with HNO2?
Ans.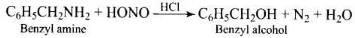
Q.41. What is the best reagent to convert nitrile to primary amine?
Ans. Reduction of nitriles with sodium alcohol or LiAlH4 gives primary amine.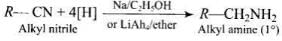
Q.42. Give the structure of ‘A’ in the following reaction.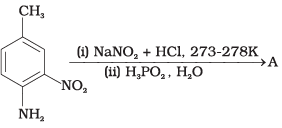 Ans.
Ans.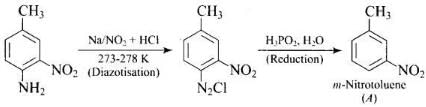
Q.43. What is Hinsberg reagent?
Ans. Hinsberg reagent is benzene sulphonyl chloride (C6H5SO2Cl).
Q.44. Why is benzene diazonium chloride not stored and is used immediately after its preparation?
Ans. Benzene diazonium chloride is very unstable. Therefore, it cannot be stored and is used immediately after its preparation.
Q.45. Why does acetylation of —NH2 group of aniline reduce its activating effect?
Ans. Acetylation of the —NH₂ group in aniline reduces its activating effect because the acetyl group (-COCH₃) withdraws electron density from the nitrogen atom through resonance and inductive effects. This decreases the ability of the nitrogen lone pair to donate electrons to the benzene ring, thereby reducing its electron-donating resonance effect (+R effect). As a result, the overall electron density on the benzene ring decreases, making it less activated toward electrophilic substitution reactions.
Q.46. Explain why MeNH2 is stronger base than MeOH?
Ans. Nitrogen is less electronegative than oxygen, therefore, lone pair of electrons on nitrogen is readily available for donation. Hence, MeNH2 is more basic than MeOH.
Q.47. What is the role of pyridine in the acylation reaction of amines?
Ans. Pyridine and other bases are used to remove the side product i.e., HCl formed during reaction mixture.
Q.48. Under what reaction conditions (acidic/basic), the coupling reaction of aryldiazonium chloride with aniline is carried out?
Ans. Coupling reaction of aryl diazonium chloride with aniline is carried out in mild acidic condition (pH = 4-5).
Q.49. Predict the product of reaction of aniline with bromine in non-polar solvent such as CS2.
Ans.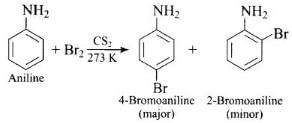
In non-polar solvent (such as CS2) the activating effect of -NH2 group is reduced (due to resonance) and hence, mono substitution occurs only at o- and p-positions.
Q.50. Arrange the following compounds in increasing order of dipole moment. CH3CH2CH3, CH3CH2NH2, CH3CH2OH
Ans. CH3CH2CH3 < CH3CH2NH2 < CH3CH2OH
Since O is more electronegative than N, therefore, dipole moment of ethyl alcohol is higher than that of ethyl amine. Propane however, has the least dipole moment since it is almost a non-polar molecule.
Q.51. What is the structure and IUPAC name of the compound, allyl amine?
Ans. The structure of allyl amine is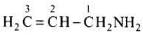 and its IUPAC name is Prop-2-en-1-amine.
and its IUPAC name is Prop-2-en-1-amine.
Q.52. Write down the IUPAC name of 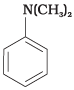
Ans. N, N-dimethylbenzenamine.
Q.53. A compound Z with molecular formula C3H9N reacts with C6H5SO2Cl to give a solid, insoluble in alkali. Identify Z.
Ans. Compound ‘Z’ with molecular formula C3H9N is an aliphatic amine which on treatment with C6H5SO2Cl gives a solid, insoluble in alkali. Therefore, the product does not have any replaceable hydrogen on the nitrogen atom. In other words, the amines (Z) must be secondary amine, i.e., Z is ethyl methylamine (C2H5NHCH3).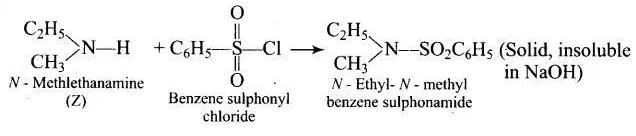
Q.54. A primary amine, RNH2 can be reacted with CH3—X to get secondary amine, R—NHCH3 but the only disadvantage is that 3° amine and quaternary ammonium salts are also obtained as side products. Can you suggest a method where RNH2 forms only 2° amine?
Ans.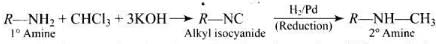
Carbylamine reaction is shown by 1° amine only which result in the replacement of two hydrogen atoms attached to N atom of -NH2 group by one carbon atom to form isocyanide. On catalytic reduction, the isocyanide will give a secondary amine w ith one methyl group.
Q.55. Complete the following reaction. Ans.
Ans.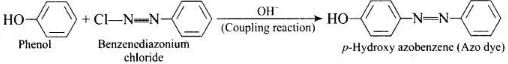
Q.56. Why is aniline soluble in aqueous HCl?
Ans. Aniline forms anilinium chloride salt with aqueous HCl which is soluble in water.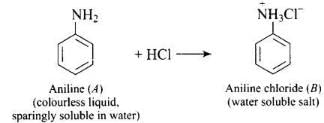
Q.57. Suggest a route by which the following conversion can be accomplished.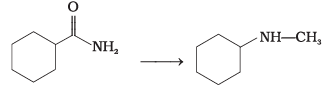 Ans.
Ans.
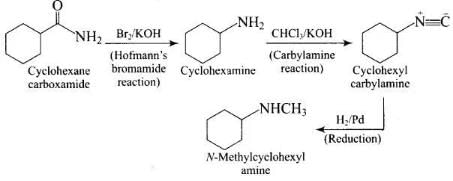
Q.58. Identify A and B in the following reaction.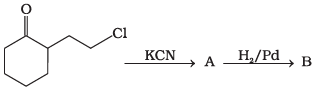 Ans.
Ans.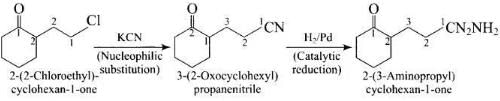
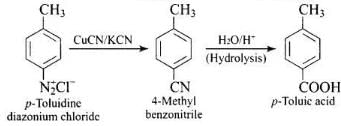
Q.59. How will you carry out the following conversions?
(1) toluene → p-toluidine
(2) p-toluidine diazonium chloride → p-toluic acid
Ans.
(1)
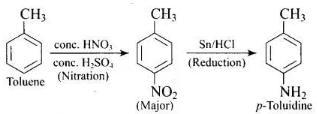
(2)
Q.60. Write following conversions:
(1) Nitrobenzene → acetanilide
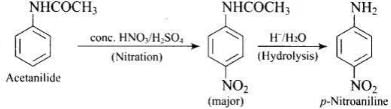
(2) Acetanilide → p-nitroaniline
Ans. (1)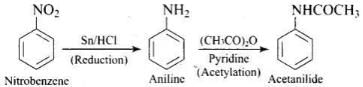
(2)
Q.61. A solution contains 1 g mol each of p-toluene diazonium chloride and p-nitrophenyl diazonium chloride. To this 1 g mol of alkaline solution of phenol is added. Predict the major product. Explain your answer.
Ans. This reaction is an example of electrophilic aromatic substitution. In alkaline medium, phenol forms phenoxide ion which is more electron rich than phenol and hence more reactive for electrophilic attack. The electrophile in this reaction is arydiazonium cation. p-Nitrophenyldiazonium cation is a stronger electrophile than p-toluene diazonium cation. Therefore, it couples preferentially with phenol.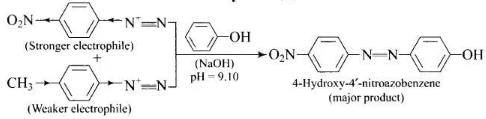
Q.62. How will you bring out the following conversion?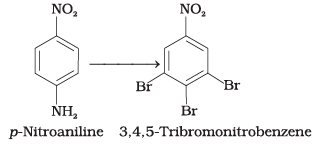
Ans.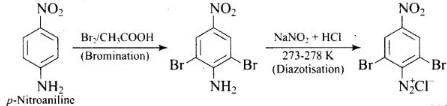
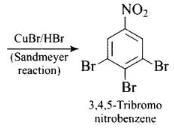
Q.63. How will you carry out the following conversion?
 Ans.
Ans.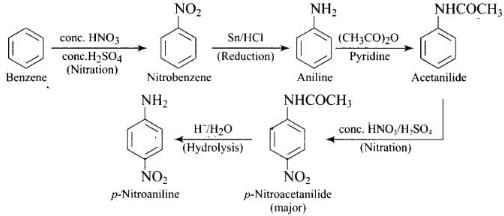
Q.64. How will you carry out the following conversion?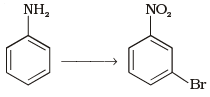 Ans.
Ans.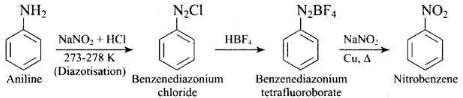
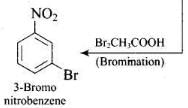
Q.65. How will you carry out the following conversions?
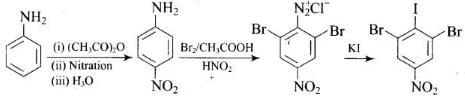
(1)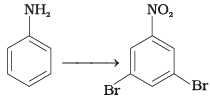
(2)
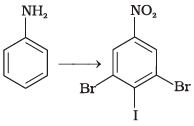
Ans.
(2)
Matching Type
Note : Match the items of Column I and Column II in the following questions.
Q.66. Match the reactions given in Column I with the statements given in Column II.
| Column I | Column II |
| (i) Ammonolysis | (a) Amine with lesser number of carbon atoms |
| (ii) Gabriel phthalimide synthesis | (b) Detection test for primary amines. |
| (iii) Hoffmann Bromamide reaction | (c) Reaction of phthalimide with KOH and R—X |
| (iv) Carbylamine reaction | (d) Reaction of alkylhalides with NH3 |
Ans. (i → d), (ii → c), (iii → a), (iv → b)
Q.67. Match the compounds given in Column I with the items given in Column II.
| Column I | Column II |
| (i) Benzene sulphonyl chloride | (a) Zwitter ion |
| (ii) Sulphanilic acid | (b) Hinsberg reagent |
| (iii) Alkyl diazonium salts | (c) Dyes |
| (iv) Aryl diazonium salts | (d) Conversion to alcohols |
Ans.(i → b), (ii → a), (iii → d), (iv → c)
| Compounds | Items |
| (i) Benzene sulphonyl chloride | Hinsberg reagent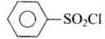 |
| (ii) Sulphanilic acid | Zwitter ion (dipolar ion)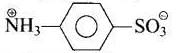 |
| (iii) Alkyl diazonium salts | Conversion to alcohols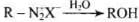 |
| (iv) Aryl diazonium salts | Dyes |
Assertion and Reason Type Questions
Note : In the following questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Q.68. Assertion : Acylation of amines gives a monosubstituted product whereas alkylation of amines gives polysubstituted product.
Reason : Acyl group sterically hinders the approach of further acyl groups.
(1) Both assertion and reason are wrong.
(2) Both assertion and reason are correct statements but reason is not correct explanation of assertion.
(3) Assertion is correct statement but reason is wrong statement.
(4) Both assertion and reason are correct statements and reason is correct explanation of assertion.
(5) Assertion is wrong statement but reason is correct statement.
Ans. (3)
Solution.
Amines on acetylation give monosubstituted product, while on alkylation gives polysubstitution product as well.
Q.69. Assertion : Hoffmann’s bromamide reaction is given by primary amines.
Reason : Primary amines are more basic than secondary amines.
(1) Both assertion and reason are wrong.
(2) Both assertion and reason are correct statements but reason is not correct explanation of assertion.
(3) Assertion is correct statement but reason is wrong statement.
(4) Both assertion and reason are correct statements and reason is correct explanation of assertion.
(5) Assertion is wrong statement but reason is correct statement.
Ans. (1)
Solution.
Hoffmann’s bromamide reaction is given by amides, not by amines. Moreover, primary amines are basic than secondary amines.
Q.70. Assertion : N-Ethylbenzene sulphonamide is soluble in alkali.
Reason : Hydrogen attached to nitrogen in sulphonamide is strongly acidic.
(1) Both assertion and reason are wrong.
(2) Both assertion and reason are correct statements but reason is not correct explanation of assertion.
(3) Assertion is correct statement but reason is wrong statement.
(4) Both assertion and reason are correct statements and reason is correct explanation of assertion.
(5) Assertion is wrong statement but reason is correct statement.
Ans. (4)
Solution.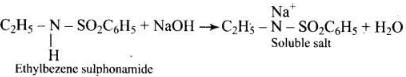
Ethylbezene sulphonamide is soluble in alkali because it has acidic hydrogen.
Q.71. Assertion : N, N-Diethylbenzene sulphonamide is insoluble in alkali.
Reason : Sulphonyl group attached to nitrogen atom is strong electron withdrawing group.
(1) Both assertion and reason are wrong.
(2) Both assertion and reason are correct statements but reason is not correct explanation of assertion.
(3) Assertion is correct statement but reason is wrong statement.
(4) Both assertion and reason are correct statements and reason is correct explanation of assertion.
(5) Assertion is wrong statement but reason is correct statement.
Ans. (2)
Solution.
N, N-diethyl benzene sulphonamide is insoluble in alkali because it has no acidic hydrogen.
Sulphonyl group attached to nitrogen atom is electron withdrawing group.
Q.72. Assertion : Only a small amount of HCl is required in the reduction of nitro compounds with iron scrap and HCl in the presence of steam.
Reason : FeCl2 formed gets hydrolysed to release HCl during the reaction.
(1) Both assertion and reason are wrong.
(2) Both assertion and reason are correct statements but reason is not correct explanation of assertion.
(3) Assertion is correct statement but reason is wrong statement.
(4) Both assertion and reason are correct statements and reason is correct explanation of assertion.
(5) Assertion is wrong statement but reason is correct statement.
Ans. (4)
Solution.
Fe + 2HCl >FeCl2 + 2[H]
Nascent hydrogen reduces nitro compounds.
FeCl2 + H2O(g) > FeO + 2HCl
Q.73. Assertion : Aromatic 1° amines can be prepared by Gabriel Phthalimide Synthesis.
Reason : Aryl halides undergo nucleophilic substitution with anion formed by phthalimide.
(1) Both assertion and reason are wrong.
(2) Both assertion and reason are correct statements but reason is not correct explanation of assertion.
(3) Assertion is correct statement but reason is wrong statement.
(4) Both assertion and reason are correct statements and reason is correct explanation of assertion.
(5) Assertion is wrong statement but reason is correct statement.
Ans. (1)
Solution.
Aromatic amines are never obtained by Gabriel phthalimide synthesis.
Q.74. Assertion : Acetanilide is less basic than aniline.
Reason : Acetylation of aniline results in decrease of electron density on nitrogen.
(1) Both assertion and reason are wrong.
(2) Both assertion and reason are correct statements but reason is not correct explanation of assertion.
(3) Assertion is correct statement but reason is wrong statement.
(4) Both assertion and reason are correct statements and reason is correct explanation of assertion.
(5) Assertion is wrong statement but reason is correct statement.
Ans. (4)
Solution.
Acetanilide is less basic than aniline because electron density of nitrogen is lowered by acetyl group.
Long Answer Type Questions
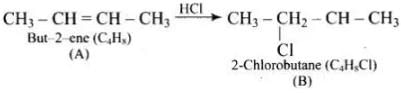
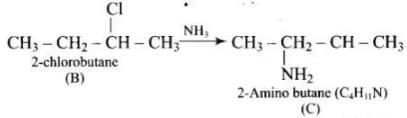
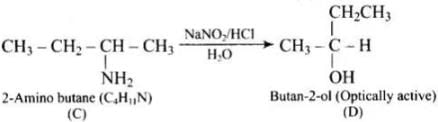
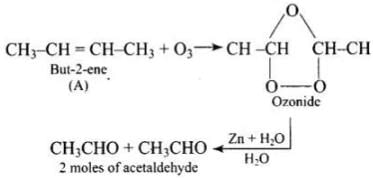
Q.75. A hydrocarbon ‘A’, (C4H8) on reaction with HCl gives a compound ‘B’, (C4H9Cl), which on reaction with 1 mol of NH3 gives compound ‘C’, (C4H11N). On reacting with NaNO2 and HCl followed by treatment with water, compound ‘C’ yields an optically active alcohol, ‘D’. Ozonolysis of ‘A’ gives 2 mols of acetaldehyde. Identify compounds ‘A’ to ‘D’. Explain the reactions involved.
Ans. (1) Addition of HCI to compound 'A' shows that compound 'A' is alkenc. Compound 'B' is C4H9Cl.
(2) Compound ‘B' reacts with NH2, it forms amine 'C'.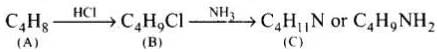
(3) 'C' gives diazonium salt with NaNO2/HCl, which yields an optically active alcohol. So, 'C’ is aliphatic amine.
(4) ‘A’ on ozonolysis produces 2 moles of CH3CHO. So. 'A' is CH3 - CH = CH - CH3 (But-2-ene).
Reactions:
(1)
(2)
(3)
(4)
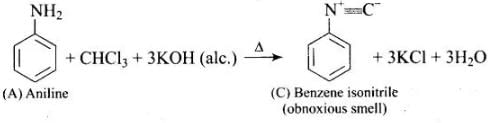
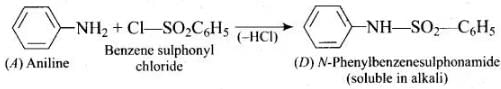
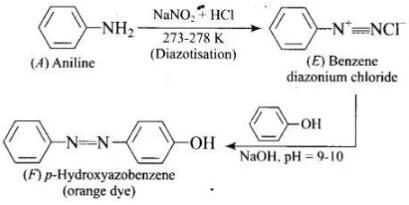
Q.76. A colourless substance ‘A’ (C6H7N) is sparingly soluble in water and gives a water soluble compound ‘B’ on treating with mineral acid. On reacting with CHCl3 and alcoholic potash ‘A’ produces an obnoxious smell due to the formation of compound ‘C’. Reaction of ‘A’ with benzenesulphonyl chloride gives compound ‘D’ which is soluble in alkali. With NaNO2 and HCl, ‘A’ forms compound ‘E’ which reacts with phenol in alkaline medium to give an orange dye ‘F’. Identify compounds ‘A’ to ‘F’.
Ans.(1)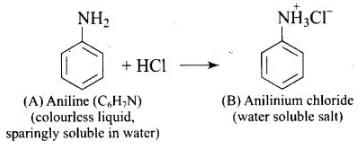
(2)
(3)
(4)
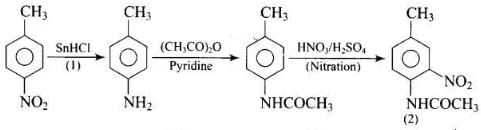
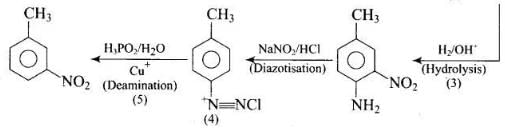
Q.77. Predict the reagent or the product in the following reaction sequence.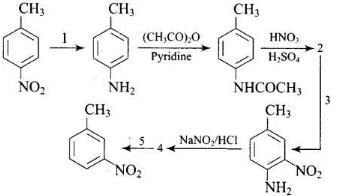 Ans.
Ans.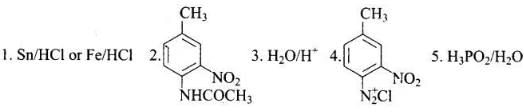
|
75 videos|278 docs|78 tests
|
FAQs on NCERT Exemplar: Amines - Chemistry Class 12 - NEET
| 1. What are amines and how are they classified? |  |
| 2. What are the common properties of amines? |  |
| 3. How do amines react with acids? |  |
| 4. What is the significance of amines in biological systems? |  |
| 5. Can you explain the methods of synthesizing amines? |  |
















