Carbon Cycle, Nitrogen Cycle, Phosphorus and Sulphur Cycle - UPSC PDF Download
Introduction
The functioning of ecosystems relies on two crucial processes: energy flow and nutrient cycling. While energy is lost as heat, nutrients, such as carbon, hydrogen, oxygen, nitrogen, and phosphorus, remain in circulation, constantly moving between living and non-living components in a biogeochemical fashion. Essential elements are recycled indefinitely, forming perfect or imperfect cycles based on replacement rates. Among the most critical nutrient cycles are the carbon and nitrogen cycles, occurring in gaseous form. Carbon, vital for life, is exchanged between the atmosphere and organisms through photosynthesis and respiration. Nitrogen, abundant in living organisms, is fixed by microbes, nitrified, ammonified, and denitrified, sustaining life.
≫ Biogeo Chemical Cycling or Nutrient Cycling
- Energy flow and nutrient circulation are the major functions of the ecosystem.
- Energy is lost as heat forever in terms of the usefulness of the system. On the other hand, nutrients of food matter never get used up. They can be recycled again and again indefinitely.
- Carbon, hydrogen, oxygen, nitrogen and phosphorus as elements and compounds makeup 97% of the mass of our bodies and are more than 95% of the mass of all living organisms.
- In addition to these, about 15 to 25 other elements are needed in some form for the survival and good health of plants and animals.
- These elements or mineral nutrients are always in circulation moving from non-living to living and then back to the non-living components of the ecosystem in a more or less circular fashion.
- This circular fashion is known as biogeochemical cycling (bio for living; geo for atmosphere).
- Among the most important nutrient cycles are the carbon nutrient cycle and the nitrogen nutrient cycle.
- There are many other nutrient cycles that are important in ecology, including a large number of trace mineral nutrient cycles.

Types of Nutrient Cycles
Based on the replacement period, a nutrient cycle is referred to as Perfect or Imperfect cycle.
- A perfect nutrient cycle is one in which nutrients are replaced as fast as they are utilized.
- Most gaseous cycles are generally considered as perfect cycles.
- In contrast sedimentary cycles are considered relatively imperfect, as some nutrients are lost from the cycle and get locked into sediments and so become unavailable for immediate cycling.
Based on the nature of the reservoir, a nutrient cycle is referred to as Gaseous or Sedimentary cycle
- Gaseous Cycle: the reservoir is the atmosphere or the hydrosphere — water cycle, carbon cycle, nitrogen cycle, etc. and
- Sedimentary Cycle: the reservoir is the earth’s crust (soluble elements mostly found in earth’s crust) — phosphorous cycle, sulphur cycle, calcium cycle, magnesium cycle etc.
≫ Carbon Cycle (Gaseous Cycle)
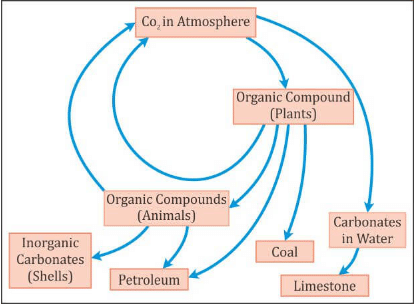

- Carbon is a minor constituent of the atmosphere as compared to oxygen and nitrogen.
- However, without carbon dioxide life could not exist because it is vital for the production of carbohydrates through photosynthesis by plants.
- It is the element that anchors all organic substances from coal and oil to DNA (deoxyribonucleic acid: the compound that carries genetic information).
- Carbon is present in the atmosphere, mainly in the form of carbon dioxide (CO2).
- Carbon cycle involves a continuous exchange of carbon between the atmosphere and organisms.
- Carbon from the atmosphere moves to green plants by the process of photosynthesis, and then to animals.
- By process of respiration and decomposition of dead organic matter, it returns to the atmosphere. It is usually a short term cycle.
- Some carbon also enters a long term cycle. It accumulates as un-decomposed organic matter in the peaty layers of marshy soil or as insoluble carbonates in bottom sediments of aquatic systems which take a long time to be released.
- In deep oceans, such carbon can remain buried for millions of years till geological movement may lift these rocks above sea level.
- These rocks may be exposed to erosion, releasing their carbon dioxide, carbonates and bicarbonates into streams and rivers.
- Fossil fuels such as coals, oil and natural gas etc. are organic compounds that were buried before they could be decomposed and were subsequently transformed by time and geological processes into fossil fuels. When they are burned the carbon stored in them is released back into the atmosphere as carbon dioxide.
≫ Nitrogen Cycle (Gaseous Cycle)
- Apart from carbon, hydrogen and oxygen, nitrogen is the most prevalent element in living organisms.
- Nitrogen is a constituent of amino acids, proteins, hormones, chlorophylls and many of the vitamins (explained in Biology NCERT).
- Plants compete with microbes for the limited nitrogen that is available in the soil. Thus, nitrogen is a limiting nutrient for both natural and agricultural ecosystems.
- Nitrogen exists as two nitrogen atoms (N2) joined by a very strong triple covalent bond (N ≡ N).
- In nature, lightning and ultraviolet radiation provide enough energy to convert nitrogen to nitrogen oxides (NO, NO2, N2O).
- Industrial combustions, forest fires, automobile exhausts and power-generating stations are also sources of atmospheric nitrogen oxides.
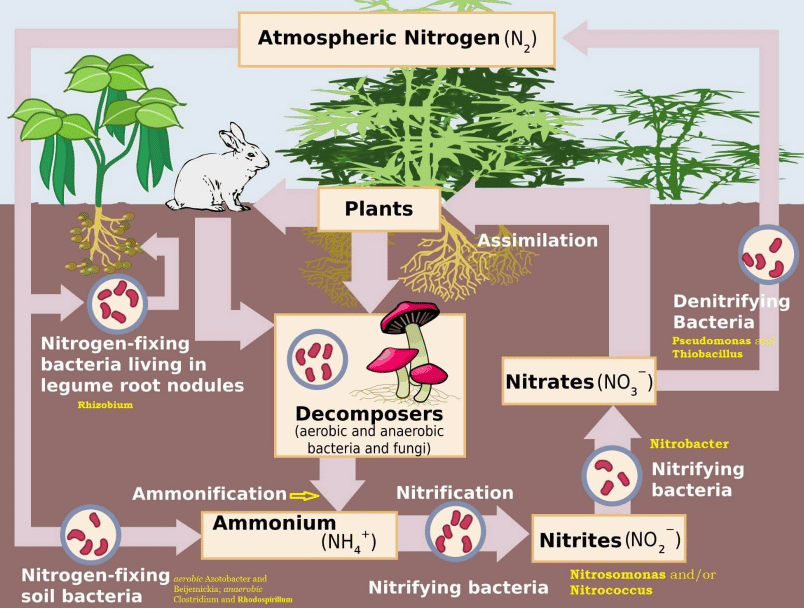
Nitrogen Fixing – Nitrogen to Ammonia (N2 to NH3)
- There is an inexhaustible supply of nitrogen in the atmosphere, but the elemental form cannot be used directly by most of the living organisms.
- Nitrogen needs to be ‘fixed’, that is, converted to ammonia, nitrites or nitrates, before it can be taken up by plants.
- Nitrogen fixation on earth is accomplished in three different ways:
(i) By microorganisms (bacteria and blue-green algae),
(ii) By man using industrial processes (fertiliser factories) and
(iii) To a limited extent by atmospheric phenomena such as thunder and lighting. - Certain microorganisms are capable of fixing atmospheric nitrogen into ammonia (NH3) and ammonium ions (NH4+).
- Ammonia (NH3) is a molecule consisting of nitrogen and hydrogen, while ammonium (NH4+) is an ion of ammonia that is formed by accepting a hydrogen ion.
- The enzyme, nitrogenase which is capable of nitrogen reduction is present exclusively in prokaryotes. Such microbes are called N2-fixers. These include:
(i) free-living nitrogen fixing bacteria (non-symbiotic nitrogen-fixing bacteria or nitrogen-fixing soil bacteria) (e.g. aerobic Azotobacter and Beijemickia; anaerobic Clostridium and Rhodospirillum),
(ii) symbiotic nitrogen-fixing bacteria (e.g. Rhizobium) living in association with leguminous plants and non-leguminous root nodule plants and
(ii) some cyanobacteria (a major source of nitrogen fixation in oceans) (blue-green algae. E.g. Nostoc, Anabaena, Spirulina etc.). - Leguminous: denoting plants of the pea family (Leguminosae), typically having seeds in pods, distinctive flowers, and root nodules containing nitrogen-fixing bacteria.
Nitrification – Ammonia to Nitrates
- Ammonium ions can be directly taken up as a source of nitrogen by some plants.
- Others absorb nitrates which are obtained by oxidising ammonia and ammonium ions.
- Ammonia and ammonium ions are oxidised to nitrites or nitrates by two groups of specialized bacteria.
(i) Ammonium ions are first oxidised to nitrite by the bacteria Nitrosomonas and/or Nitrococcus.
(ii) The nitrite is further oxidized to nitrate with the help of the bacterium Nitrobacter. - These steps are called nitrification. These nitrifying bacteria are chemoautotrophs (they use inorganic chemical energy sources to synthesise organic compounds from carbon dioxide).
- The nitrate thus formed is absorbed by plants and is transported to the leaves.
- In leaves, it is reduced to form ammonia that finally forms the amine group of amino acids, which are the building blocks of proteins. These then go through higher trophic levels of the ecosystem.
- Nitrification is important in agricultural systems, where fertiliser is often applied as ammonia.
- Conversion of this ammonia to nitrate increases nitrogen leaching because nitrate is more water-soluble than ammonia.
- Nitrification also plays an important role in the removal of nitrogen from municipal wastewater.
- The conventional removal is nitrification, followed by denitrification.


Ammonification – Urea, Uric Acid to Ammonia
- Living organisms produce nitrogenous waste products such as urea and uric acid (organic nitrogen).
- These waste products, as well as dead remains of organisms, are converted back into inorganic ammonia and ammonium ions by the bacteria. This process is called ammonification.
- Some of this ammonia volatilizes and re-enters the atmosphere, but most of it is converted into nitrate by soil bacteria.
Denitrification – Nitrate to Nitrogen
- Nitrate present in the soil is reduced to nitrogen by the process of denitrification.
- In the soil as well as oceans there are special denitrifying bacteria (Pseudomonas and Thiobacillus), which convert the nitrates/nitrites to elemental nitrogen.
- This nitrogen escapes into the atmosphere, thus completing the cycle.
(i) Step 1: N2 Fixing ⇒ Nitrogen → Ammonia or Ammonium Ions
(ii) Step 2: Nitrification ⇒ Ammonia or Ammonium Ions → Nitrite → Nitrate
(iii) Step 3: Ammonification ⇒ Dead Matter + Animal Waste (Urea, Uric Acid) → Ammonia or Ammonium Ions
• Most of the ammonia escapes into the atmosphere. Rest is Nitrified (Step 2) to nitrates.
• Some of the nitrates is available for plants. Rest is Denitrified (Step 4).
(iv) Step 4: Denitrification ⇒ Nitrate → Nitrogen - The amount of nitrogen fixed by man through the industrial process has far exceeded the amount fixed by the Natural Cycle.
- As a result, nitrogen fixed by man has become a pollutant which can disrupt the balance of nitrogen. It may lead to Acid rain, Eutrophication and Harmful Algal Blooms.
Phosphorus Cycle (Sedimentary cycle)

- Phosphorus plays a central role in aquatic ecosystems and water quality.
- Unlike carbon and nitrogen, which come primarily from the atmosphere, phosphorus occurs in large amounts as a mineral in phosphate rocks and enters the cycle from erosion and mining activities.
- This is the nutrient considered to be the main cause of excessive growth of rooted and free-floating microscopic plants (phytoplankton) in lakes (leads to eutrophication).
- The main storage for phosphorus is in the earth’s crust.
- On land, phosphorus is usually found in the form of phosphates.
- By the process of weathering and erosion, phosphates enter rivers, streams and finally oceans.
- In the ocean, phosphorus accumulates on continental shelves in the form of insoluble deposits.
- After millions of years, the crustal plates rise from the seafloor and expose the phosphates on land.
- After more time, weathering will release them from rock, and the cycle’s geochemical phase begins again.
Sulphur Cycle (Sedimentary cycle)
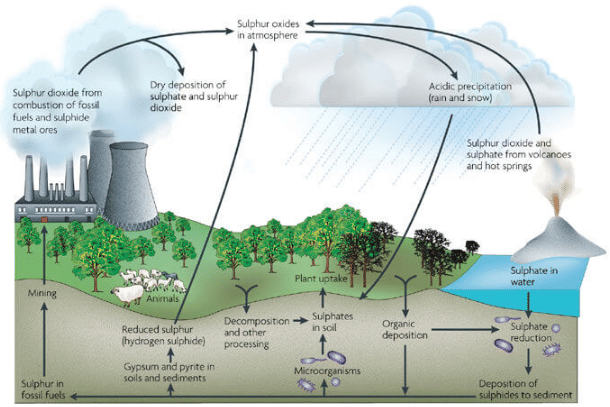

- The sulphur reservoir is in the soil and sediments where it is locked in organic (coal, oil and peat) and inorganic deposits (pyrite rock and sulphur rock) in the form of sulphates, sulphides and organic sulphur.
- It is released by weathering of rocks, erosional runoff and decomposition of organic matter and is carried to terrestrial and aquatic ecosystems in salt solution.
- The sulphur cycle is mostly sedimentary except two of its compounds, hydrogen sulphide (H2S) and sulphur dioxide (SO2), which add a gaseous component.
- Sulphur enters the atmosphere from several sources like volcanic eruptions, combustion of fossil fuels (coal, diesel etc.), from the surface of the ocean and gases released by decomposition.
- Atmospheric hydrogen sulphide also gets oxidised into sulphur dioxide.
- Atmospheric sulphur dioxide is carried back to the earth after being dissolved in rainwater as weak sulphuric acid (acid rain).
- Whatever the source, sulphur in the form of sulphates is taken up by plants and incorporated through a series of metabolic processes into sulphur bearing amino acid which is incorporated in the proteins of autotroph tissues. It then passes through the grazing food chain.
- Sulphur bound in a living organism is carried back to the soil, to the bottom of ponds and lakes and seas through excretion and decomposition of dead organic material.



















