Important Thermodynamics Formulas for JEE and NEET
Thermodynamic processes :
- Isothermal process: T = constant
dT = 0
ΔT = 0 - Isochoric process: V = constant
dV = 0
ΔV = 0 - Isobaric process: P = constant
dP = 0
ΔP = 0 - Adiabatic process: q = 0
or heat exchange with the surrounding = O(zero)
IUPAC Sign convention about Heat and Work :
Work done on the system = Positive
Work done by the system = Negative
1st Law of Thermodynamics
ΔU = (U2 - U1) = q + w
Law of equipartion of energy :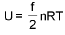 (only for ideal gas)
(only for ideal gas)
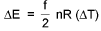
where f= degrees of freedom for that gas. (Translational + Rotational)
f = 3 formonoatomic
= 5 for diatomic or linear polyatmic
= 6 for non - linear polyatmic
Calculation of heat (q):
Total heat capacity:
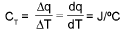
Molar heat capacity :
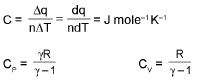
Specific heat capacity (s):
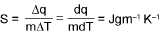
WORK DONE (w) :
Isothermal Reversible expansion/compression of an ideal gas :
W = - nRT In (Vf/Vi)
Reversible and irreversible isochoric processes.
Since dV = 0
So dW = - Pext . dV = 0.
Reversible isobaric process:
W = P (Vf - Vi)
Adiabatic reversible expansion :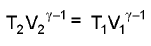
Reversible Work: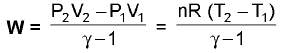
Irreversible Work :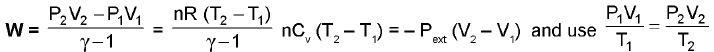
Free expansion - Always going to be irrerversible and since Pext = 0
so dW = -Pext..dV = 0
If no. heat is supplied q = 0
then ΔE = 0 so ΔT = 0.
Application of 1st Law :
ΔU = ΔQ + ΔW ⇒ ΔW = -P ΔV
∴ ΔU = ΔQ -PΔV
Constant volume process
Heat given at constant volume = change in internal energy
∴du = (dq)v
du = nCvdT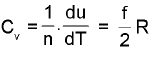
Constant pressure process:
H ≡ Enthalpy (state function and extensive property)
H = U + PV
⇒ Cp - Cv = R (only for ideal gas)
Second Law Of Thermodynamics : fo r a spontaneous process.
fo r a spontaneous process.
Entropy (S):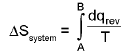
Entropy calculation for an ideal gas undergoin a process :
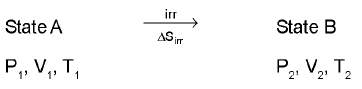
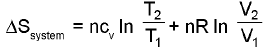 (only for an ideal gas)
(only for an ideal gas)
Third Law Of Thermodynamics :
The entropy of perfect crystals of all pure elements & compounds is zero at the absolute zero of temperature.
Gibb’s free energy (G) : (State function and an extensive property)
Criteria of spontaneity:
(i) If ΔGsystem is (-ve) < 0 ⇒ process is spontaneous
(ii) If ΔGsystem is > 0 ⇒ process is non spontaneous
(iii) lf ΔGsystem = 0 = 3 system is at equilibrium.
Physical interpretation of ΔG :
The maximum amount of non-expansional (compression) work which can be performed.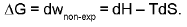
Standard Free Energy Change (ΔG°) :
1. ΔG° = -2.303 RTIog10K
2. At equilibrium ΔG = 0.
3. The decrease in free energy (-ΔG) is given as :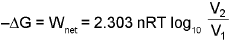
4. 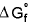 for elemental state = 0
for elemental state = 0
5. 
Thermochemistry:
Change in standard enthalpy 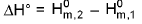
= heat added at constant pressure. = CPΔT.
If Hproducts > Hreactants
- Reaction should be end other micas we have to give extra heat to reactants to get these converted into products and if
Hproducts > Hreactants - Reaction will be exothermic as extra heat content of reactants will be released during the reaction. Enthalpy change of a reaction :

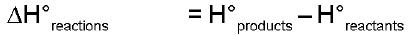
= positive - endothermic
= negative - exothermic
Temperature Dependence Of ΔH : (Kirchoff's equation):
For a constant volume reaction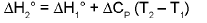
where ΔCP = Cp (products) - Cp (reactants).
For a constant volume reaction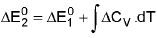
Enthalpy of Reaction from Enthalpies of Formation :
The enthalpy of reaction can be calculated by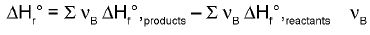 is the stoichiometric coefficient.
is the stoichiometric coefficient.
Estimation of Enthalpy of a reaction from bond Enthalpies :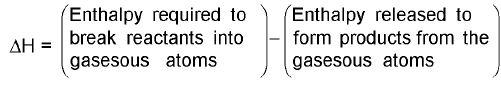
Resonance Energy: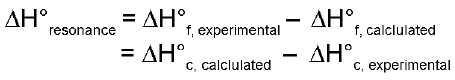
|
75 videos|278 docs|78 tests
|






















