Points to Remember: Adsorptions & Colloids | Physical Chemistry PDF Download
Points to be Remembered
1. Some Important Terms :
- Adsorption: Accumulation of a substance (gas) on the surface of another material (solid) over the bulk is known as adsorption.
- Adsorbate: The material which is getting adsorbed is known as adsorbate.
- Adsorbent: The material on which the adsorption take place is known as adsorbent.
- Desorption: The reverse process of adsorption is known as desorption.
2. Signs of ΔG, ΔH and ΔS for the Adsorption of a Gas on Solid:
The adsorption of a gas on solid occurs spontaneously. Hence, the change in Gibbs free energy will be negative i.e. ΔG = - ve.
As adsorption causes a decrease in the degrees of freedom of the gas molecules, this process involves a decrease in the entropy i.e. the change in entropy will be negative i.e. ΔS = - ve.
Hence, the adsorption process is entropically unfavourable. But, as the process occurs spontaneously, the change in enthalpy for the process must ve highly negative i.e. ΔH = - ve.
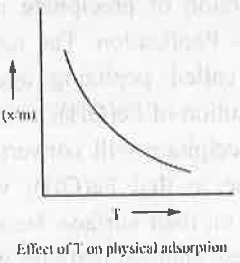
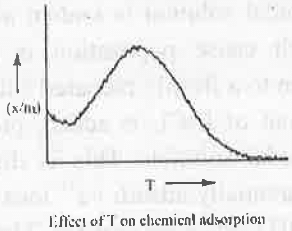
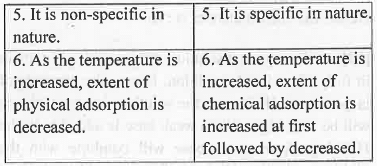
3. Types of Adsorption: Adsorption is classified into two category - Physical Adsorption and Chemical Adsorption. The basic differences are given below: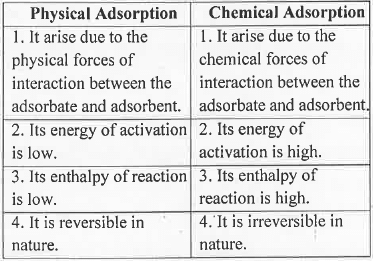
 4. Factors Affecting Adsorption: The following factors affects the adsorption process.
4. Factors Affecting Adsorption: The following factors affects the adsorption process.
I. Nature of Adsorbate: Adsorption process depends on the extent of interaction between the adsorbate and adsorbent. Greater is the interaction, higher will be the extent of adsorption. In general adsorbate is gas. Hence, greater is the intermolecular force present in the gas, higher will be the chance of accumulation of the gas on the solid surface. Hence, higher will be the adsorption.
II. Nature of Adsorbent: In general, adsorbent is solid. Hence, greater is the surface area of the solid, higher will be extent of the accumulation of gas on solid surface. Hence, higher will be the adsorption.
III. Effect of Temperature:
- Physical Adsorption: In case of physical adsorption, the change in enthalpy is negative i.e. it involves the evolution of heat. Now, according to Le Chateliar's principle if temperature is further increased, the equilibrium will be disturbed. To reestablished the equilibrium the reaction will occur to that direction which involves absorption of heat i.e. the reaction will occur in a reverse direction. Hence, on increasing temperature, the rate of physical adsorption will decrease.
- Chemical Adsorption: In case of chemical adsorption high activation energy is needed. Hence, increasing temperature will at first provide activation energy making the adsorption process faster. After that further increasement of temperature will decrease the chemical forces. Hence, the rate of chemical adsorption will decrease.


5. Freundlich Adsorption Isotherm: Freundlich Adsorption Isother is defined as:
θ = x/m = K.p1/n........ (1)
where, x and m are the amount of adsorbate and amount of adsorbent respectively.
A plot of θ vs. p will be as follows: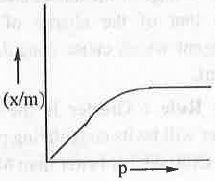 Taking log on both sides of the equation (1), we have:log(x/m) = logK + (l/n).logp
Taking log on both sides of the equation (1), we have:log(x/m) = logK + (l/n).logp
Hence, a plot of log(x/m) vs. logp will be a straight line with slope (1/n) and intercept logK.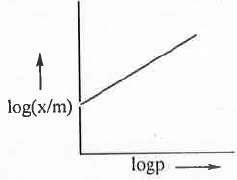 6. Langmuir Adsorption Isotherm:
6. Langmuir Adsorption Isotherm:
Langmuir adsorption isotherm is defined as : θ = ap /(l+ bp ) ....... (1)
where, a and b are the constants which depends on the nature of adsorbate, nature of adsorbent and temperature.
- I. At low pressure region: When p is low, (1+bp) ≅ 1. Hence, θ = ap or, θ α p1 i.e. fractional coverage linearly dependent on pressure at low pressure.
- II. At high pressure region: When p is high, (1+bp) ≅ bp. Hence, θ = ap/bp or, θ α p° i.e. fractional coverage almost independent on pressure at high pressure.
Assumptions for Langmuir Adsorption theory:
- All the adsorption sites are equivalent.
- There exist a dynamic equilibrium between free gas and adsorbed gas.
- The probability for fresh adsorption is independent of occupancy at the neighbouring sites.
- Also, it is only valid for the monolayer formation.
7. Thomas Graham is known as the Father of Colloidal Chemistry
8. Difference Between True Solution and Colloidal Solution : True Solution Colloidal
Solution.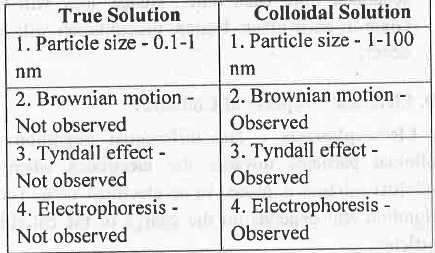 9. Lyophobic vs. Lyophilic Colloids:
9. Lyophobic vs. Lyophilic Colloids:
- Definitions: Colloids in which the dispersed phase ' has strong affinity for dispersion medium is known as Lyophilic colloid while colloid in which the dispersed phase has no affinity for dispersion medium.
- Stability: Lyophilic colloid has greater stability than the lyophobic colloid.
Stability of Lyophobic Colloids: Lyophobic colloids are stable due to the following reasons.
- Lyophobic colloids adsorb preferentially those ions to which it is chemically similar. As a result of preferential adsorption of ions, colloids become charged. Hence, these will repel each other and will not come closer to each other. Hence, precipitation will not occur. This is one of the reasons for the stability of lyophobic colloidal particles.
- Lyophobic colloids undergo double layer formation - fixed layer and diffuse layer. In the first layer i.e. fixed layer adsorption of positive or negative charge occurs due to chemical interactions. In the second layer i.e. opposite charges are adsorbed due to Columbic interaction. A potential difference exist between these two layers which is known as Zeta Potential which is responsible for the stability of the Lyophobic colloids.
- Brownian motion does not allow the colloidal particles for settle down making colloid stable.
Stability of Lyophilic Colloids: Lyophilic colloids are stable due to the following reasons.
- Due to preferential adsorption of ions.
- Due to double layer formation.
- Brownian motion.
- Due to strong affinity of dispersed phase particles towards dispersion medium, colloidal particles becomes surrounded by dispersion medium molecules. Hence, the colloidal particles become separated from each other. Hence, they will not come to each other. Hence, precipitation will not occur.
10. Electrical Property of Colloids:
I. Electrophoresis: The differential migration of colloidal particles towards the electrodes when a colloidal solution is placed in an electrical field. Their migration will depends on the charge of the colloidal particles.
II. Electro-osmosis: The differential migration of dispersion medium towards the electrodes when a colloidal solution is placed in an electrical field. Their migration will also depends on the charge of the dispersion medium.
Electrophoresis and electro-osmosis phenomenon proved that dispersed phase and dispersion mediums are charged. But, the colloidal solutions are as a whole neutral.
11. Tyndall effect: When a beam of light from a source is passed through a colloidal solution and the path of the light is seen at a right angle to the path through which the light is passing then the path of the light gets observed. This is due to that the colloidal particles will at first absorb the light and then it will scatter the absorb light. It is independent on the charge o f the particle to which the light get scatter.
12. Brownian Motion: When the colloidal particles are seen through ultra-microscope, it is observed that the particles are not stand still. These are in random and ceaseless motion. This is known as Brownian motion.
This is due to the unequal bombardment of dispersion medium particles on dispersed phase particles. Due to Brownian motion colloids becomes stable.
13. Peptization: The conversion of precipitate into colloidal solution is known as Peptization. The agent which cause peptization is called peptizing agent. When to a freshly prepared solution of Fe(OH)3 a small amount of FeCI3 is added, precipitate w ill convert to colloidal solution. This is due to that Fe(OH)3 will preferentially adsorb Fe3+ ions on their surface. Hence, Fe(OH)3 becomes charged. These charged particles will repel each other. Hence, these will breaks down into smaller particles causing formation of colloidal solution.
14. Coagulation: The conversion of colloidal solution into precipitate by adding suitable electrolyte is called coagulation. The charge of the added electrolyte should be opposite to that of the charge of the colloidal particles. The agent which cause coagulation is called coagulating agent.
Schulze-Hardy Rule: Greater is the charge of an electrolyte higher will be its coagulating power.
For coagulating CdS , Al3+ is better than Mg2+ and Na+.
|
83 videos|142 docs|67 tests
|
FAQs on Points to Remember: Adsorptions & Colloids - Physical Chemistry
| 1. What is adsorption? |  |
| 2. How does adsorption differ from absorption? |  |
| 3. What are some examples of adsorption? |  |
| 4. What are colloids? |  |
| 5. How are colloids different from solutions? |  |

|
Explore Courses for Chemistry exam
|

|