Chemical Bonding: Definition & Types | Chemistry Class 11 - NEET PDF Download
What is a Chemical Bond?
Chemical Bonding refers to the formation of a chemical bond between two or more atoms, molecules, or ions to give rise to a chemical compound. These chemical bonds are what keep the atoms together in the resulting compound.
 Chemical Bond
Chemical Bond
- The matter is made up of one or different types of elements. Under normal conditions, no other element exists as an independent atom in nature, except noble gases.
- However, a group of atoms is found to exist together as one species having characteristic properties. Such a group of atoms is called a molecule.
- Obviously, there must be some force that holds these constituent atoms together in the molecules. The attractive force that holds various constituents (atoms, ions, etc.) together in different chemical species is called a chemical bond.
Chemical Bonding
- Atoms are usually not capable of free existence, therefore a group of atoms of the same or different elements exist together as one species and have characteristic properties.
- A molecule will only be formed if it is more stable and has lower energy than individual atoms.
- Chemical Bonding refers to the formation of a chemical bond between two or more atoms, molecules, or ions to give rise to a chemical compound. These chemical bonds are what keep the atoms together in the resulting compound.
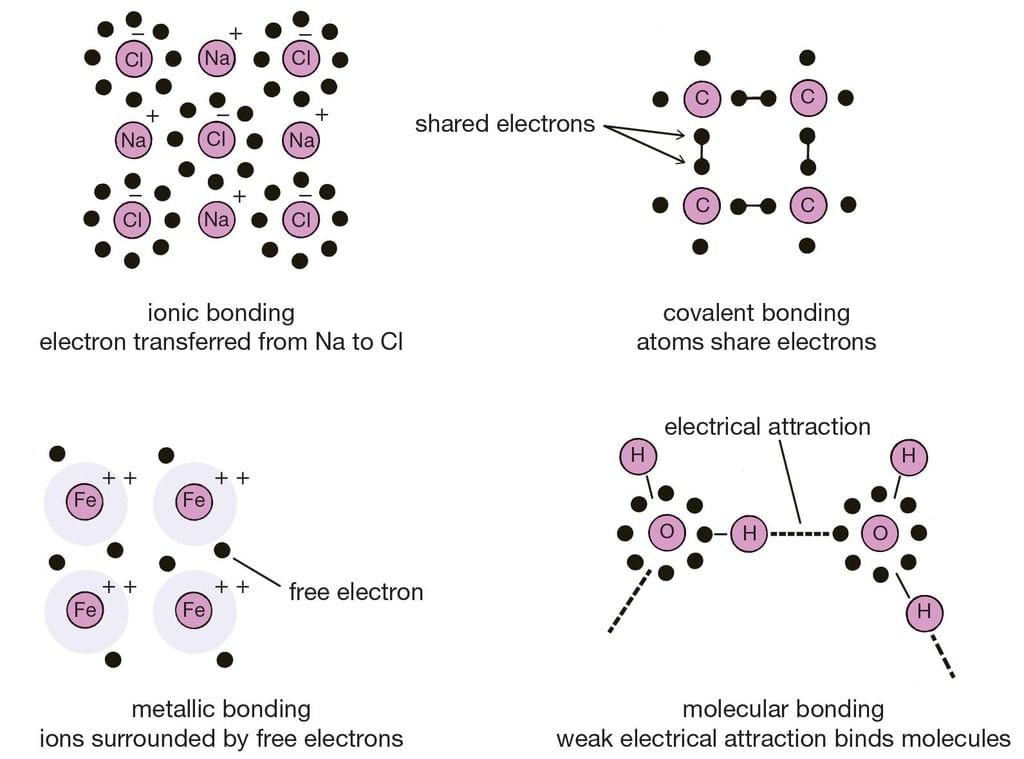 Different types of Chemical Bonding
Different types of Chemical Bonding
Properties of Chemical Bond
- A force that acts between two or more atoms to hold them together as a stable molecule.
- It is a union of two or more atoms involving the redistribution of electrons among them. This process is accompanied by a decrease in energy.
- The decrease in energy ∝ Strength of the bond.
- Therefore molecules are more stable than atoms.
Cause of Chemical Combination
1. Tendency to acquire minimum energy
- When two atoms approach each other. The nucleus of one atom attracts the electrons of another atom.
- According to the quantum theory when two atoms of an element approach each other then there will be a force of attraction as well as the force of repulsion between the bonded atoms.
- The minimum distance where these forces become equal is called bond formation condition (equilibrium state) and atoms of the elements get stabilized by bond formation phenomena by lowering their energy.
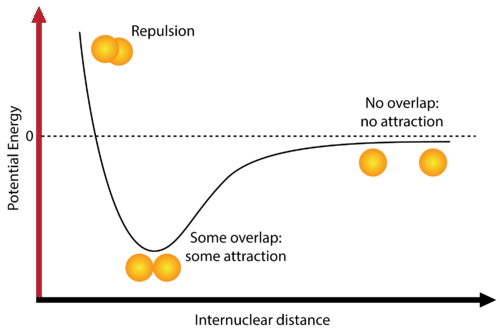
- If the net result is attraction, the total energy of the system (molecule) decreases, and a chemical bond forms. No chemical bonding is possible if the net result is repulsion.
- So, Attraction ∝ 1/energy ∝ Stability.
The potential energy curve of the H₂ molecule illustrates how the potential energy varies with the internuclear distance. The key points to note are:
- At very short distances, repulsive forces dominate, resulting in high potential energy.
- At an optimal intermediate distance (equilibrium bond length), attractive forces dominate, leading to a minimum potential energy and a stable H₂ molecule.
- Beyond this equilibrium distance, the potential energy increases as the attractive forces weaken.
Note: Bond formation is an exothermic process.
2. Tendency to acquire noble gas configuration
- Inert gas elements do not participate, as they have stable electronic configuration and hence minimum energy. (Stable electronic configuration: 1s2 or ns2np6)
- Atom combines to acquire noble gas configuration, i.e., they try to attain either 2 electrons (when only one energy shell) or 8 electrons in their outermost energy level which is of maximum stability and hence of minimum energy.
- Only outermost shells, i.e. ns np and (n -1) d, electrons participate in bond formation.
 Noble Gases
Noble Gases
Kossel-Lewis Approach To Chemical Bond
- Albrecht Kössel and Gilbert Lewis were the first to explain the formation of chemical bonds successfully in the year 1916. They explained chemical bonding based on the inertness of noble gases.
- Lewis had a model for atoms where he imagined a central part with a positive charge called the "Kernel," consisting of the nucleus and inner electrons.
- The outer part could hold up to eight electrons. He thought these eight electrons would arrange themselves at the corners of a cube around the Kernel.
- For example, sodium's single outer electron would be at one corner, while a noble gas would have all eight corners filled. This grouping of eight electrons is seen as a very stable arrangement.
Lewis Octet Rule
- Every atom has a tendency to complete its octet.
- Hydrogen has the tendency to complete its duplet.
- To acquire inert gas configuration atoms lose or gain electron(s) or share electron(s).
- The tendency of atoms to achieve eight electrons in their outermost shell is known as the Lewis octet rule.
- The octet rule can be observed in the bonding between the carbon and oxygen atoms in a carbon dioxide molecule, as illustrated via a Lewis dot structure below.
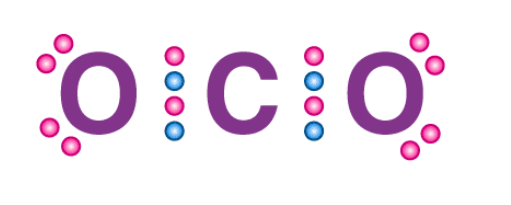 Structure of CO2
Structure of CO2
- The shared electrons fulfill the valency requirements of both the bonded atoms. Thus, it can be noted that both the oxygen atoms and the carbon atoms have an octet configuration in the CO2 molecule.
Explanation of Kossel Lewis Approach
- In 1916, Kossel and Lewis succeeded in giving a successful explanation based upon the concept of an electronic configuration of noble gases about why atoms combine to form molecules.
- Atoms of noble gases have little or no tendency to combine with each other or with atoms of other elements. This means that these atoms must have stable electronic configurations.
- Due to the stable configuration, the noble gas atoms neither have any tendency to gain or lose electrons and, therefore, their combining capacity or valency is zero.
- They are so inert that they even do not form diatomic molecules and exist as monoatomic gaseous atoms.
Limitations of Octet Rule
1. Incomplete Octet Molecules
- Also known as Electron deficient molecules or Hypovalent molecules, in which, the compound's octet is not complete in the outermost orbit of the central atom.
Examples: Halides of IIIA groups, BF3, AlCl3, BCl3; Hydride of III A/13th group, BeCl2 (4 electrons), ZnCl2 (4 electrons), Ga(CH3)3 (6 electrons) etc.
 Incomplete Octet
Incomplete Octet
2. Expansion of Octet
- Also known as Electron Efficient Molecules or Hypervalent molecules, in which, compound's central atom has more than 8 electrons in outermost orbits.
Examples: PCI5, SF6, IE7, the central atom P, S and I contain 10, 12, and 14 electrons respectively.
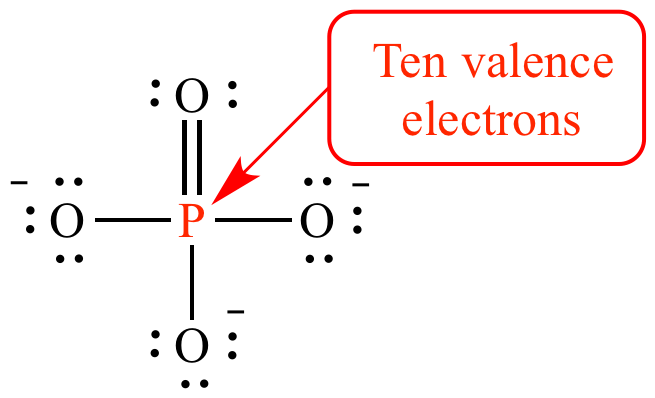 Expansion of Octet
Expansion of Octet
3. Pseudo Inert Gas Configuration
- Cations of transition metals, which contain 18 electrons in the outermost orbit.
Examples: Ga+3, Cu+, Ag+, Zn+2, Cd+2, Sn+4, Pb+4 etc. - Electronic configuration of Ga: 1s2, 2s22p6, 3s23p63d10, 4s24p1
Electronic configuration of Ga+3: 1s2, 2s2 2p6, 3s23p63d10 (18 electrons)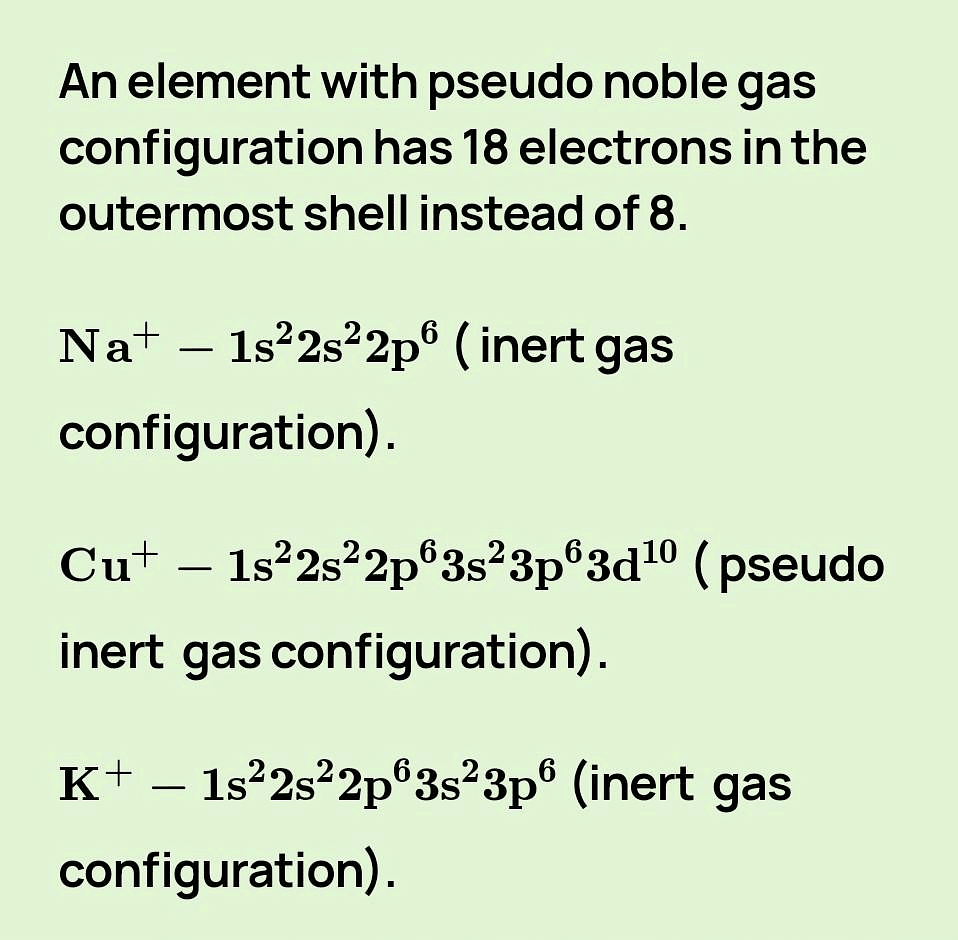
4. Odd Electron Molecules
- Central atom has an unpaired electron or odd number (7 electrons, 11 electrons, etc) of electrons in their outermost shell.
Examples: NO, NO2 ClO2, ClO3 etc.
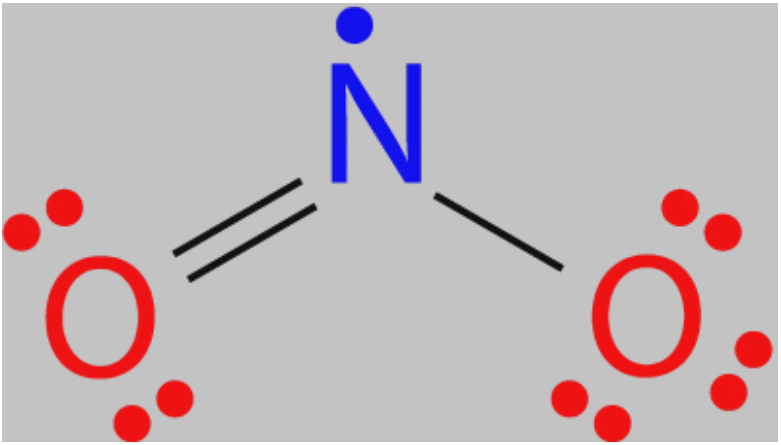 Electron Dot Structure of NO
Electron Dot Structure of NO
Types of Chemical Bonding
When substances participate in chemical bonding and yield compounds, the stability of the resulting compound can be gauged by the type of chemical bonds it contains.
The type of chemical bonds formed vary in strength and properties.
There are 4 primary types of chemical bonds which are formed by atoms or molecules to yield compounds. These types of chemical bonds include:
- Ionic Bonds
- Covalent Bonds
- Hydrogen Bonds
- Polar Bonds
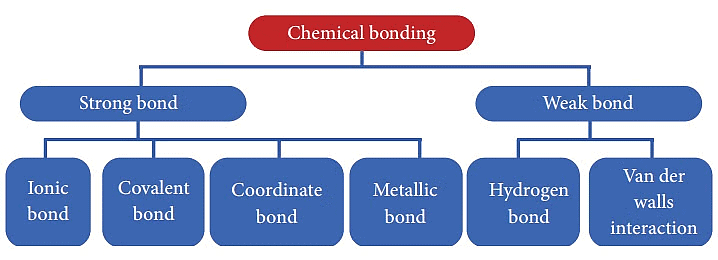 Types of Chemical Bonds
Types of Chemical Bonds
These types of bonds in chemical bonding are formed from the loss, gain, or sharing of electrons between two atoms/molecules.
Strong Bonds
1. Ionic Bond
When a bond is formed by complete transference of electrons from one atom to another so as to complete their outermost orbits by acquiring 8 electrons (i.e., octet) or 2 electrons (i.e., duplet) in the case of hydrogen, lithium, etc., and hence acquire the stable nearest noble gas configuration, the bond formed is called ionic bond or electrovalent bond.
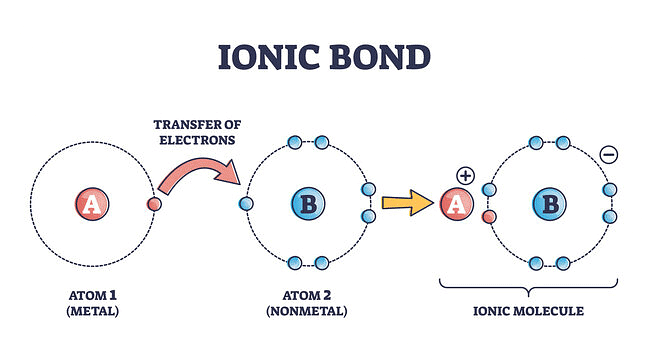 Ionic Bond
Ionic Bond
Explanation of Ionic Bond
Atoms are electrically neutral. Therefore, they possess an equal number of protons and electrons. On losing an electron, an atom becomes positively charged since now the number of protons exceeds the number of electrons.
A → A+ + e-
On the other hand, in the case of an atom, gaining the electron, the number of electrons exceeds the number of protons and thus the atom becomes negatively charged.
B + e- → B-
The oppositely charged particles formed above attract each other by electrostatic forces of attraction. The bond thus formed is known as an electrovalent or ionic bond.
Example: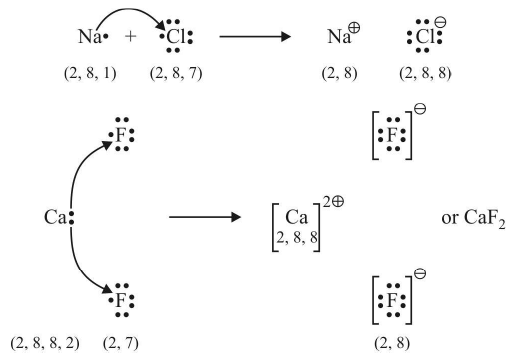
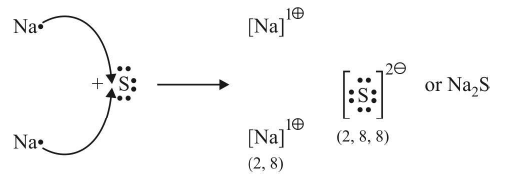
Electrovalency
The number of electrons lost or gained during the formation of an electrovalent linkage is termed the electrovalency of the element.
For example, sodium and calcium lost 1 and 2 electrons respectively and so their valencies are 1 and 2. Similarly, chlorine and oxygen gain 1 and 2 electrons respectively, so they possess an electrovalency of 1 and 2. In other words, valency is equal to the charge on the ion.
Factors governing the formation of ionic bonds
(i) Ionisation Enthalpy (Ionization Energy)
The ionisation enthalpy of any element is the amount of energy required to remove an electron from the outermost shell of an isolated atom in the gaseous phase to convert it into a gaseous positive ion.
It is clear that the lesser the ionization enthalpy, the easier the removal of an electron, i.e., the formation of a positive ion, and hence greater the chances of the formation of an ionic bond.
Ionization enthalpy (I.E.) of alkali metals (i.e., group I elements) is low, hence they have more tendency to form positive ions.
(ii) Electron Gain Enthalpy (Electron Affinity)
Electron affinity or Electron gain enthalpy of an element is the enthalpy change that takes place when an extra electron is added to an isolated atom in the gaseous phase to form a gaseous negative ion.
The higher is the electron affinity, the more energy released and the stabler will be the negative ion produced. Consequently, the probability of the formation of ionic bonds will be enhanced.
Halogens possess high electron affinity. So the formation of their negative ions is very common, e.g., in the case of chlorine, electron affinity is +348 kJ/mole, i.e.,
Cl (g) + e– → Cl– + 348 kJ/mole
or E.Z. = + 348 kJ mol–1
(iii) Lattice Enthalpy (Lattice Energy)
In the formation of ionic compounds, the positively charged ions combine with negatively charged ions to form the compound.
A+ (g) + B– (g) → A+ B– (s)
The energy released when the requisite number of gaseous positive and negative ions combine to form one mole of the ionic compound is called lattice enthalpy.
Born-Haber cycle: It is an indirect method to calculate the lattice energy of an ionic compound. For example, the lattice energy of sodium chloride can be calculated as follows.
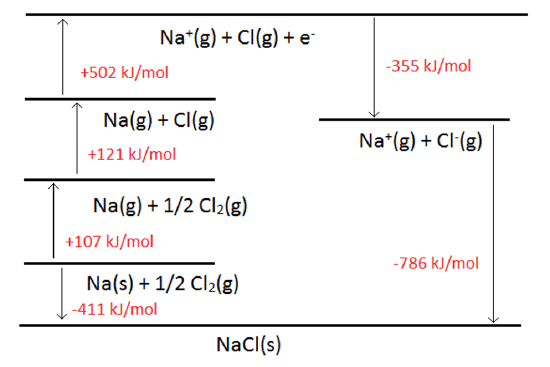
Now, according to Hess's law,
= S + IE1 + D/2 - EA1 - U
Where S is the enthalpy of sublimation of metal (Na), IE1 is the first ionization energy of sodium, D is the bond dissociation energy of Cl molecule, EA1 is the first electron affinity of Cl, U is the lattice energy of NaCl(s) and DHformation is the enthalpy of formation of NaCl.
Characteristics of Ionic Compounds
1. Physical State
These compounds usually exist in the solid state.
2. Crystal Structure
X-ray analysis of the ionic compounds shows that they exist as ions and not as molecules. These ions are arranged in a regular pattern in the three-dimensional space to form a lattice.
The pattern of arrangement, however, depends upon the size and charges of the ions. For example, in the case of sodium chloride, each sodium ion is surrounded by six chloride ions and each chloride by six sodium ions, thus giving rise to a three-dimensional octahedral crystal structure (figure). The formula of an ionic compound merely indicates the relative number of ions present.
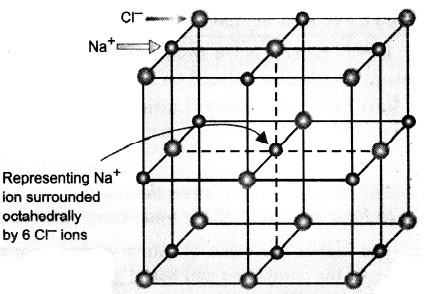 Crystal Structure of NaCl
Crystal Structure of NaCl
3. High melting and boiling points
Ionic compounds possess high melting and boiling points.
This is because ions are tightly held together by strong electrostatic forces of attraction and hence a huge amount of energy is required to break the crystal lattice.
4. Solubility
Electrovalent compounds are soluble in solvents like water which are polar in nature and have high dielectric constant.
It is due to the reason that the polar solvent interacts with the ions of the crystals and further the high dielectric constant of the solvent (i.e., the capacity of the solvent to weaken the forces of attraction) cuts off the force of attraction between these ions. Furthermore, the ions may combine with the solvent to liberate energy called the hydration enthalpy which is sufficient to overcome the attractive forces between the ions.
Non-polar solvents like carbon tetrachloride, benzene, etc. having low dielectric constants are not capable of dissolving ionic solids. Hence, ionic solids are soluble in polar solvents and insoluble in non-polar solvents.
5. Electrical conductivity
Ionic compounds are good conductors of electricity in solution or in the molten state. In solution or molten state, their ions are free to move. As the ions are charged, they are attracted towards electrodes and thus act as carriers of electric current.
6. Ionic Reactions
The reactions of the ionic compounds are, in fact, the reactions between the ions produced in solution. As the oppositely charged ions combine quickly, these reactions are, therefore, quite fast.
[e.g. Na+ Cl– (aq) + Ag+ NO3– (aq) → AgCl (s) + NaNO3 (aq)]
2. Covalent Bond
The bond formed between the two atoms by the mutual sharing of electrons between them so as to complete their octets or duplets in the case of elements having only one shell is called a covalent bond or covalent linkage and the number of electrons contributed by each atom is known as covalency.
Example:
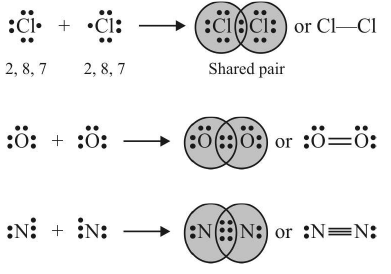
 Conditions for the formation of covalent bonds
Conditions for the formation of covalent bonds
- Electronegativity: An atom will not transfer the electron or electrons to the other atom if the electronegativity difference between the two. atoms are zero or very small (less than 1.6). Such atoms prefer to. share electrons, i.e., form covalent bonds.
- Shortage of Electrons: When both, atoms are short in electrons in the valency shell in comparison to. stable noble configuration, then such atoms complete the outermost shell by sharing electrons. Except for hydrogen which has one electron in valence shell, such atoms have 5, 6 or 7 valency electrons. The non-metals of group V A, VIA and VIlA satisfy this condition.
Factors Influencing the Formation of Covalent Bond:
1. Electron Affinity:
A covalent bond is generally favored between the two atoms if both atoms have high electron affinity.
2. Ionisation Energy:
The ionization energy of both the atoms participating in bonding should be high.
3. Atomic Size:
The atomic size of the atoms forming covalent bonds should be smaller. The smaller the atomic radii of atoms, the stronger the covalent bond will be. For example, the H-H bond is stronger than the Cl-Cl bond which in turn is stronger than the Br-Br bond.
4. Electronegativity:
The electronegativities of both then atoms should be high. The difference in electronegativities between the two atoms should be minimal.
Covalency
It is defined as the number of electrons contributed by an atom of the element for sharing with other atoms as to achieve noble gas configuration. It can also. be defined as the number of covalent bonds formed by the atom of the element with other atoms.
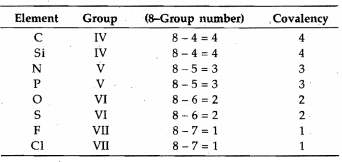
Generally, the covalency of an element is equal to. the total number of unpaired electrons in s- and p-orbitals of the valency shell.
 These four elements do not possess d-orbitals in their valency shell. However, the elements having vacant d-orbitals in their valency shell-like P, S, CI, Br, and I, show
These four elements do not possess d-orbitals in their valency shell. However, the elements having vacant d-orbitals in their valency shell-like P, S, CI, Br, and I, show
variable covalency by increasing the number of unpaired electrons under excited conditions, i.e., unpairing the paired orbital and shifting the electrons to. vacant d-orbitals.
Example:
Draw the Lewis dot structure of the HCN molecule.
Sol. Step-1: Total number of valence electrons in HCN = 1 + 4 + 5 = 10 (1H = 1, 6C = 2 ,4, 7N = 2, 5)
Step 2: Skeletal structure is HCN (C is least electronegative).
Step 3: Putting one shared pair of electrons between H and C and one between C and N, and the remaining as lone pairs, we have
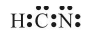
In this structure, the duplet of H is complete but octets of C and N are not complete. Hence, multiple bonding is required between and N. Octets of C and N will be complete if there is triple bond between C and N. Thus,
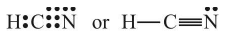 Example:
Example:
Draw the Lewis dot structure of CO32– ion.
Sol. Step-1: Total number of valence electrons of CO3 = 4 + 3 × 6 = 22(6C = 2, 4, 8O = 2, 6)
Step-2: Total number of electrons to be distributed in CO32– = 22 + 2 (for two units -ve charge) = 24
Step-3: The skeletal structure of CO3 is
Step-4: Putting one shared pair of electrons between each C and O and completing the octets of oxygen, we have

In this structure, octet of C is not complete. Hence, multiple bonding is required between C and one of the O–atoms. Drawing a double bond between C and one O-atom serves the purpose:
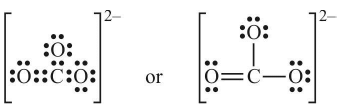
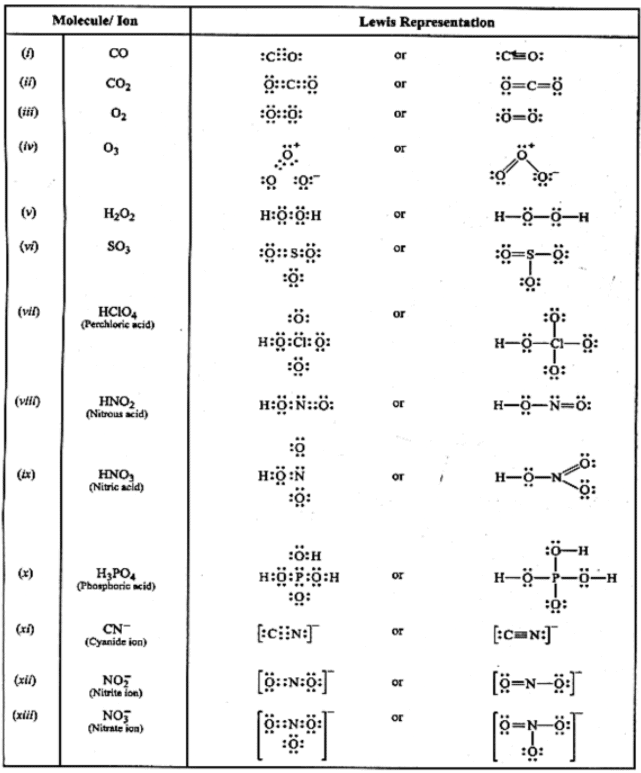
Table: Lewis structures of some typical molecules and ions
Fajan's Rule
This rule is used to decide the relative ionic & covalent character of a molecule. A molecule is predominantly covalent if
(I) Smaller the size of the cation.
(II) larger the size of the anion.
(III) greater the charge on cation and anion.
(IV) ion does not have an inert gas configuration but it possesses a pseudo inert gas configuration (18 electrons in the ultimate shell).
(I)
(II)
(III)
(IV) CuCl and NaCl
[Cu ] = [Ar]3d10 ; [Ne ] = [Ne]
Cations with 18-electron shells (pseudo inert gas configuration) have greater polarising power than 8-electron shell (inert gas configuration) ions with the same charge and size. Thus, CuCl is more covalent than NaCl.
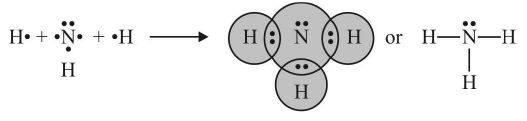
3. Coordinate Bond or Dative Bond
- When both the electrons for sharing between two atoms are contributed by one atom only the bond formed is known as a coordinate bond or dative bond. In terms of orbital theory, the coordinate covalent bond is formed by overlapping between empty and completely filled atomic orbitals.
- The atom donating the pair of electrons is called the donor and the atom which accepts the pair of electrons is called the acceptor. The compounds containing coordinate bonds are known as coordination compounds.
- The bond is represented by an arrow () pointing head towards the acceptor.
Once the coordinate bond is formed it is indistinguishable from a covalent bond. Examples:
1. Formation of SO2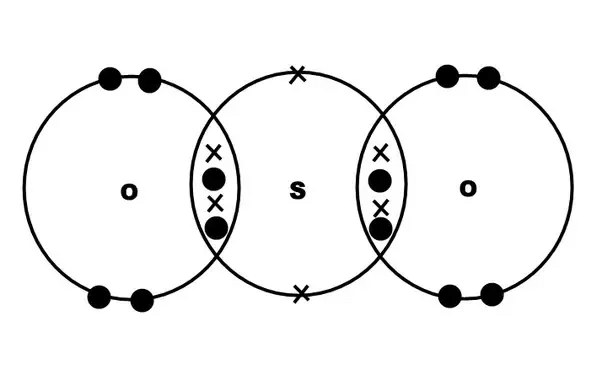 SO2 Formation2. Formation of SO3
SO2 Formation2. Formation of SO3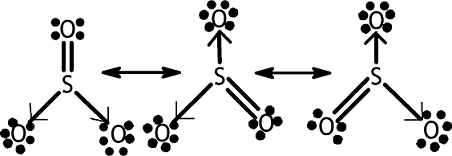 Formation of SO33. Formation of Hydroxonium ion
Formation of SO33. Formation of Hydroxonium ion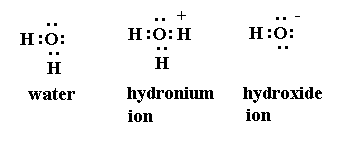 4. NH3 and BF3 form addition product by Coordinate covalent bond
4. NH3 and BF3 form addition product by Coordinate covalent bond
4. Metallic Bond
- The constituent particles of metallic solids are metal atoms that are held together by the metallic bond.
- A metal atom is supposed to consist of two parts, valence electrons and the remaining part (the nucleus and the inner shells) called the kernel.
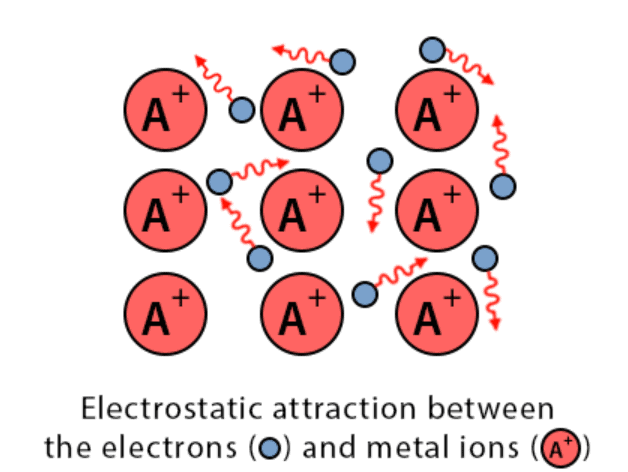 Metallic Bond
Metallic Bond
- The kernels of metal atoms occupy the lattice sites while the space between the kernel is occupied by valence electrons.
- Due to small ionization energy, the valence electrons of metal atoms are not held by the nucleus firmly. Therefore, the electrons leave the field of influence of one kernel and come under the influence of another kernel.
- Thus the electrons are not localized but are mobile. The simultaneous attraction between the kernels and the mobile electrons that hold the kernel together is known as a metallic bond. This model is known electron sea model.
Weak Bonds
Weak Bonds
1. Hydrogen Bonding
- When a hydrogen atom linked to a highly electronegative atom (like F, O, or N) comes under the influence of another strongly electronegative atom, then a weak bond is developed between them which is called a hydrogen bond.
- It is represented by the dotted line as follows:
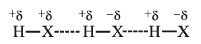
- As a result of hydrogen bonding, the H–atom links the two electronegative atoms simultaneously, one by a covalent bond and the other by a hydrogen bond. Hence it is said to form a hydrogen bridge.
- It is merely a strong electrostatic attractive force and not a normal chemical bond. It is very weak (strength about 2-10 Kcal/mol).
Conditions for Hydrogen bonding
(a) The molecule must contain a highly electronegative atom linked to H-atom. The higher the electronegativity, the more is the polarization of the molecule.
(b) The size of the electronegative atom should be small. The smaller the size the greater is the electrostatic attraction.
Types of Hydrogen bonding
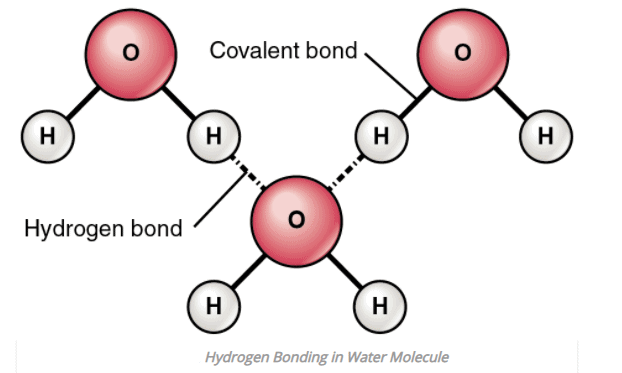
1. Intermolecular Hydrogen bonding
- When hydrogen bonding takes place between different molecules of the same or different compounds, it is called intermolecular hydrogen bonding.
- Examples: HF, H2O, ROH (same compound) water-alcohol, water ammonia (different compound), etc.
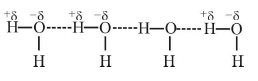
2. Intramolecular hydrogen bonding
- The hydrogen bonding takes place within a molecule itself.
- It takes place in compounds containing two groups such that one group contain the H-atom linked to an electronegative atom and the other group contains a highly electronegative atom linked to a lesser electronegative atom.
- The bond is formed between the H-atom of one group with the more electronegative atom of the other group.
- Example: O-Nitrophenol, O-Niholeny aldehyde
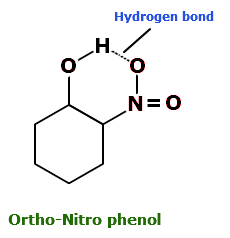 Example of Intramolecular H-bonding
Example of Intramolecular H-bonding
2. Vander Waal’s Forces
- This type of attractive force occurs in the case of non-polar molecules such as H2, O2, Cl2, CH4, CO2, etc.
- The existence of weak attractive forces among the nonpolar molecules was first proposed by Dutch scientist J.D. Vander Waal.
- Vander Waal force ∝ molecular weight ∝ Boiling point
Types of Vander Waal’s force
1. Ion-Dipole attraction: This force is between an ion such as Na+ and a polar molecule such as HCl.
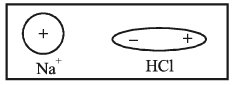
2. Dipole-Dipole attraction: It is again in between two polar molecules such as HF and HCl.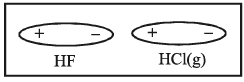
3. ion-induced dipole attraction: In this case, a neutral molecule is induced by an ion as a dipole.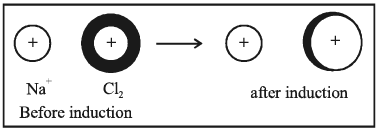
4. Dipole-induced dipole attraction - In this case, a neutral molecule is induced as a dipole by another dipole.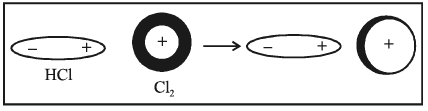
5. Induced dipole-induced dipole attraction or London dispersion force between two non-polar molecules as in Cl2, He etc.
Note:
The relative strength of various bonds is as follows:
Ionic bond > Covalent bond > Metallic bond > H-bond > Vander waal bond.
3. London Dispersion Forces
Another form of chemical bonding is caused by London dispersion forces. These forces are weak in magnitude.
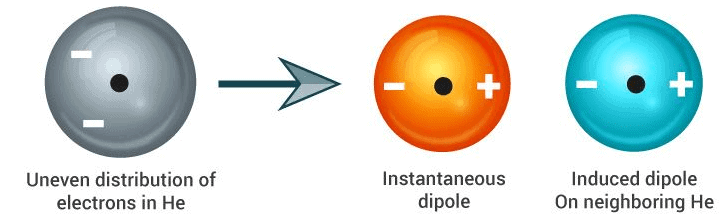
These forces occur due to a temporary charge imbalance arising in an atom. This imbalance in the charge of the atom can induce dipoles on neighbouring atoms. For example, the temporary positive charge on one area of an atom can attract the neighbouring negative charge.
|
121 videos|348 docs|74 tests
|
FAQs on Chemical Bonding: Definition & Types - Chemistry Class 11 - NEET
| 1. What is the cause of chemical combination? |  |
| 2. What is the Kossel-Lewis approach to chemical bond? |  |
| 3. What are the types of chemical bonding? |  |
| 4. What are examples of strong bonds in chemical bonding? |  |
| 5. What are weak bonds in chemical bonding? |  |

















