Methods of Preparation of Haloalkane & Haloarenes | Organic Chemistry for NEET PDF Download
Haloalkanes and Haloarenes can be prepared from other organic compounds by numerous methods. Different methods of preparation include conversion of alcohols to alkyl halides, the addition of halogens to alkenes, and hydrohalogenation of alkenes. The preparation techniques were so reliable and efficient that they became an inevitable part of industrial chemistry.
There are primarily 4 different types of preparation techniques of Haloalkanes and Haloarenes.
- Alcohols
- Hydrocarbons
- Alkenes by addition of hydrogen halides and halogens
- Halogen exchange reaction.
So let’s learn about the methods of preparation of Haloalkanes and Haloarenes.
Preparation of Haloalkanes:
Preparation of Haloalkanes:
1. From Alcohols:
1. From Alcohols:
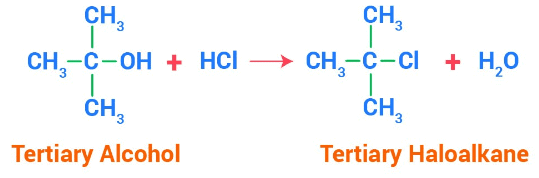
Alkyl halides can easily be prepared from alcohols upon the addition of halides. In this reaction, the hydroxyl group of alcohol is replaced with the halogen atom attached to the other compound involved. This reaction requires a catalyst for primary and secondary alcohols whereas it doesn’t require any catalyst for tertiary alcohols.
The general reaction looks like this: ROH + HX → RX + H2O
a. Reaction with HCl:
b. Reaction with Bromine: CH3CH2OH + HBr → CH3CH2Br + H2O
c. Reaction with Phosphorous Halide:
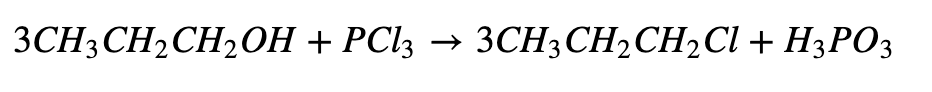
d. Reaction with Thionyl Chloride:
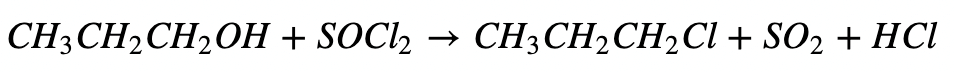
2. From Alkenes:
The addition of hydrogen halides to alkenes follows either Markovnikov’s rule or exhibit the Kharash effect. All the electrophilic addition reactions of alkenes following the Markovnikov rule are known as Markovnikov addition reactions. A general example of such reaction is given below:
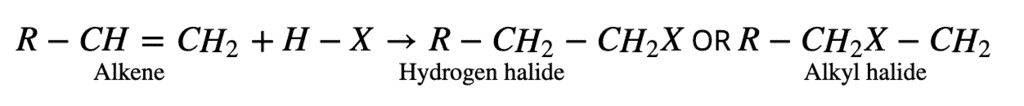
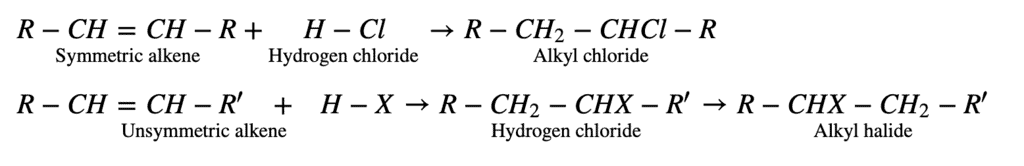
3. From Free Radical Halogenation:
In free radical halogenation, we get a mixture of mono-substituted, di-substituted, tri-substituted, and even tetra-substituted halo-alkanes (alkyl halides). Since we require only one type of alkyl halide and not all in the form of a mixture, So this method is not used.
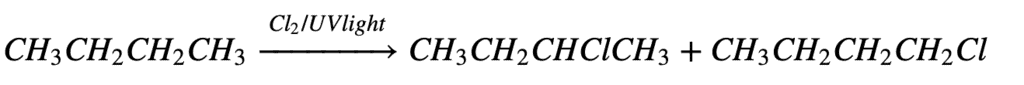
4. From Halogen Exchange:
a. Finkelstein Reaction:
In this reaction, an alkyl chloride or alkyl bromide reacts with sodium iodide in acetone to form alkyl iodides.
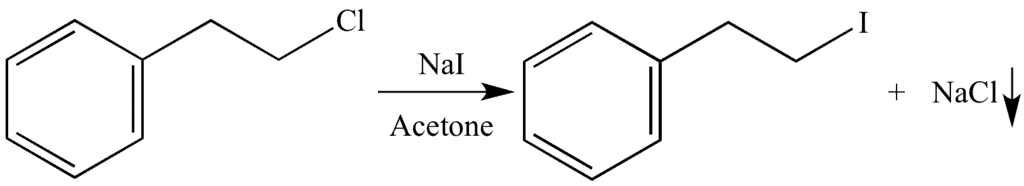
The solubility difference of alkyl halides in acetone is used for driving the reaction in the forward direction. We know that sodium iodide is soluble in acetone but NaCl or NaBr are insoluble. Therefore, they precipitate out in the reaction which is easy to remove from the reaction mixture.
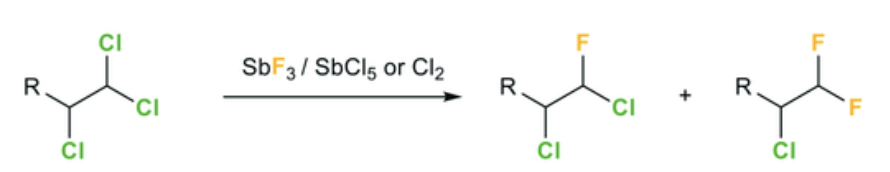
b. Swartz Reaction:
In this reaction, alkyl fluorides formation is possible by heating of Alkyl fluorides RBr/RCl. The reaction is carried out in the presence of metallic fluoride such as SbF3, Hg2F2, AgF, CoF2.
Preparation of Haloarenes:
1. From hydrocarbons by Electrophilic Substitution Reactions
Aryl halides can be prepared by electrophilic aromatic substitution of arenes with halogens in the presence of a Lewis acid.
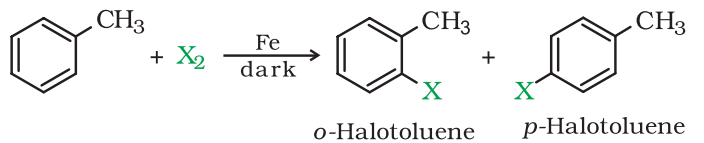
Mechanism of Electrophilic Substitution Reaction:
In the above reaction, two different isomers of the aryl chlorides are formed. They are Ortho and para isomer. The π-electron in the benzene ring attacks the Cl+ electrophile to produce an intermediate complex. However, the H+ bond from the intermediate complex moves in order to compensate for the positive charge of the carbon atom.
Thus the reaction forms two different isomers of the product-ortho and para. The melting points of both the isomer differ significantly. And para-isomer has a higher boiling point than ortho-isomer. Therefore, they can be easily separated from each other.
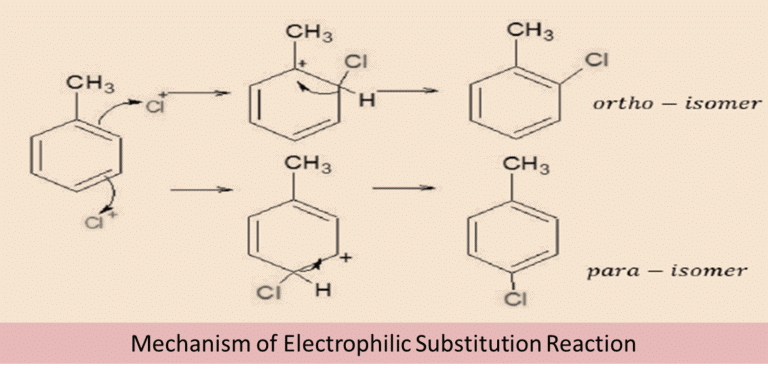
2. From amines by Sandmeyer’s reaction:
Sandmeyer’s Reaction is a two-step method that includes:
- Diazonium salt formation
- Diazonium salt reaction with a cuprous halide (Cu2X2)
Primary aromatic amine reacts with sodium nitrite in the presence of cold mineral acid to form the diazonium salt. In this case, HNO2 is prepared within the reaction by reacting sodium nitrite and HX at a temperature of 273-278K.
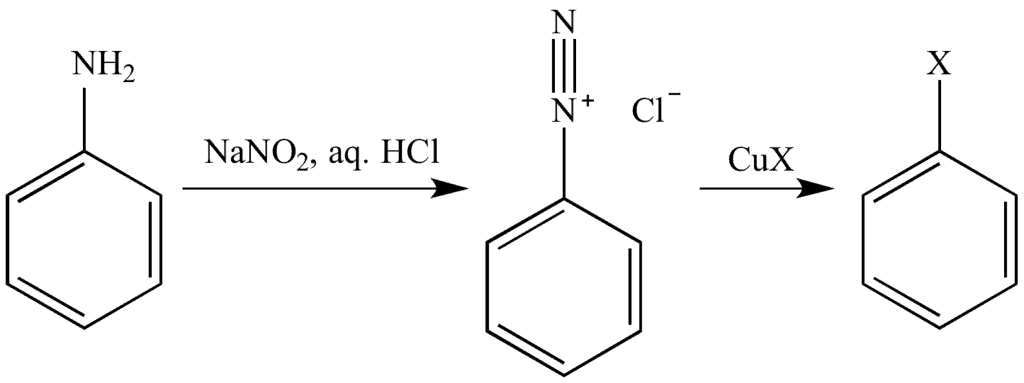
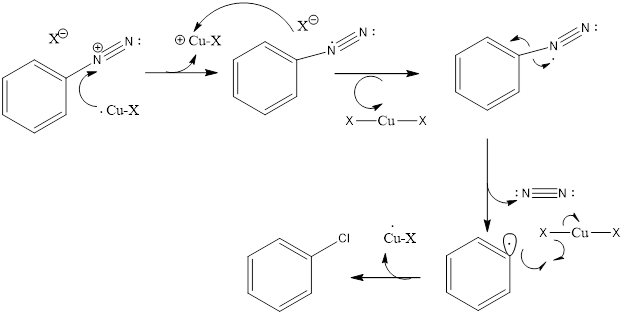
Mechanism of the Sandmeyer’s Reaction:
In the first step: NaNO2 + HCl → HNO2 + NaCl
The HNO2 formed in the presence of H+ undergo protonation to form NO+ as the electrophile. The lone pair of the atom from the primary amine will react with the electrophile to form an intermediate compound which further gives diazonium salt after elimination of H2O. In the second step, the diazonium salt reacts with cuprous halide to form the respective aryl halide
|
54 videos|125 docs|136 tests
|
FAQs on Methods of Preparation of Haloalkane & Haloarenes - Organic Chemistry for NEET
| 1. What is the general method for preparing haloalkanes from alcohols? |  |
| 2. Can all alcohols be converted into haloalkanes using this method? |  |
| 3. What are some common halogens used for the preparation of haloalkanes from alcohols? |  |
| 4. Can haloalkanes be prepared directly from alkanes? |  |
| 5. What are some applications of haloalkanes? |  |





















