Indicators, Acids, Bases & Salts | Chemistry for JEE Main & Advanced PDF Download
| Table of contents |

|
| Indicators |

|
| Acids and Bases |

|
| Buffer Solution |

|
| Ionization of Acid & Bases |

|
| pH Scale and Acidity |

|
| Hydrolysis of Salts: Introduction |

|
Indicators
Broadly defined, as indicator is substance which is used for the visual detection and determination of a specific constituent present in any sample. The visual observation used is primarily that of colour, but observations of fluorescence and turbidity are also used.Indicators under this general definition include all reagents which can be used in colorimetry flurimetry and turbidimetry. It is convenient, therefore, to define on indicator in a more limited way, as substance which is used for the visual detection of the completion of a particular reaction that is for the end point of a titration.
Acid-Base Indicators :- Acid- base indicator are organic substances which have one colour in acid solution while an altogether different colour in alkaline solution.
Theories of Acid-Base Indicators :-
Two important theories have been put forwarded to explain their behaviour:
(I) Ostwald's theory (1891) : According to this theory:
(i) Acid- base indicators are weak organic acids or bases.
(ii) They have different colours in ionised and non-ionised states i.e.
Hln H+ + ln-
(one colour) (different colour)
(iii) The colour of the indicator depends on the relative proportions of the unionised indicator molecules and its ions.
On the basis of above postulates. Ostwald explained the action of phenophthalein, methyl orange, methyl red and other acid-base indicators.
(a) Action of Phenolphthalein:- Phenolphthalein is a weak acid (HPh) and is almost unionised. Its unionised molecules are colourless whilst on ionisation give colourless H ions and pink coloured Ph- ions.
HPh H+ + Ph-
(colourless) (colourless) (pink)
In the presence of acid due to increase in the concentration of common H ions, the dissociation of HPh is suppressed and thus the solution becomes colourless.
On the other hand, the addition of strong bases (like NaOH, KOH), however, the OH- ions produced from them combine with the H ions from the phenonphthalein to form feebly ionised water. The equilbrium (i) is thus disturbed and more of the phenolphthalein ionises to produce Ph- ions. The latter combine with Na ions to form the strongly ionised sodium salt NaPh and hence remains in the ionic state giving pink coloured Ph- ions.
(b) Methyl orange :- It is a weak base and can be represented as MeOH. Its undissociated molecule is yellow while gives red coloured Me ions on dissociation,
MeOH Me+ + OH-
(yellow) (Red) (colourless)
If a base (i.e., OH- ions) is added to the indicator, the OH- ions will suppress the ionisation of the indicator. Hence, the indicator will remain yellow in an alkali. However, if a small excess of acid (say, HCl) is added, the latter will force the equilibrium to the right by removing OH- ions to form H2O. This will result in the formation of red coloured Me ions in the solution.
MeOH Me+ + OH-
(yellow) (Red) (colourless)
HCl Cl- + H+
MeCl H2O
(Highly ionized) (Unionised)
(II) Modern Quinoid Theory :- Main postulates of this theory are:
(i) The indicators used in acid-alkali titrations are aromatic organic compounds which are equilibrium mixtures of at least two tautomeric forms, ordinarily one form is benzenoid while the other is quinoid.
(ii) The two forms have different colours. The quinoid form is usually deeper in colour than the benzenoid form. Out of these one form exists in acidic solution while other in alkaline solution.
(iii) Change in pH causes the transition of benzenoid form to quinoid form and vice versa and consequently a change in colour.
This theory explains the action of phenolphthalein, methyl orange and other acid-base indicators.
(a) Action of Phenolphthalein:- Phenolphthalein is an acidic indicator undergoing the following transformation:
In alkaline medium, OH- ions combine with H ions produced by the indicator, the equilibrium shifts to the right producing pink colour. In acid medium the dissociation of the organic acid is suppressed, the equilibrium shifts to the left and the solution becomes colourless.
(b) Action of Methyl Orange
Methyl orange is a basic indicator. More correctly it is an amphoteric compound containing both acidic group -SO3H and basic group -N(CH3)2. The quinoid form (red) combines with the OH-ions in alkaline medium favouring the formation of yellow.
(c) Action of Methyl Red
Methyl red is a basic indicator and the colour change takes place according to the following scheme:
Acid-Base Indicators with pH range of colour change
Indicator | Colour in | Colour in alkali | pH range of colour change |
Methyl orange | Red | Yellow | 3.2 - 4.4 |
Bromo-cresol green | Yellow | Blue | 3.8 - 5.4 |
Methyl red | Yellow | Red | 4.8 - 6.0 |
Bromothymol blue | Yellow | Blue | 6 - 7.5 |
Phenol red | Yellow | Red | 6.3 - 8.4 |
Phenolphthalein | Colourless | Pink | 8.2 - 10 |
Thymol blue | Yellow | Blue | 8 - 9.6 |
Thymolphthalein | Colourless | Blue | 9. 4 - 10.6 |
Choice of Indicators:-
At the equivalence point of acid- base titration there occurs a sudden jump in pH of the solution. An indicator, the pH range of colour change of which falls within this limit, is suitable and is used in that titration. No sudden pH jump of the solution in the titration of weak acid with weak base occurs and so not indicator is suitable for this titration.
Acids and Bases
Earlier Definitions of Acids and Bases
Earlier definitions of acids and bases was given by Robert Boyle, who classified them on the basis of their properties. According to him, acids are the substance which have sour taste. turns blue litmus red. liberate hydrogen with metals, conduct electricity in aqueous solution and neutralise bases.
Bases are the substance which have bitter taste, turns red litmus blue, soapy to touch, conduct electricity in aqueous solution and neutralise acids.
Arrhenius Concept of Acids and Bases
- Acid is a chemical substance which dissociates in aqueous solution to give hydrogen ions (H+) or hydronium ions (H3O+).
- Base is a chemical substance which dissociates in aqueous solution to give hydroxyl ions (OH–).
- Arrhenius theory fails to explain the acidic and basic behaviour in non-aqueous solutions. It cannot explain the acidic character of A1Cla. BFa and basic character of NH3, PH3, etc.
Bronsted Concept of Acids and Bases
Acid is a chemical substance that can donate a proton (H+) to some other substance and a base is a chemical substance that can accept a proton from other substance. Thus, an acid is a proton donor (protongenic) and a base is proton acceptor (protopbilic).
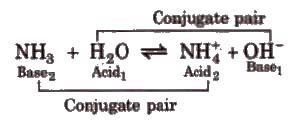
Strong acid has weak conjugate base and weak acid has strong conjugate basco Strong base has weak conjugate acid and weak base has strong conjugate acid.
HClO4 is the strongest while HCN is the weakest hydracid known.
CsOH is the strongest base known.
Amphoteric or arnphiprotic substance or ampholytes are the substance which act as an acid as well as a base, e.g.• water acts as an acid with NH3 and a base with acetic acid.
The order of acidic strength of some acids is
HClO4 > HBr> H2SO4 > HCl> HNO3
Greater the Ka value of an acid (or lesser the pKa), stronger is the acid. Similarly. greater the Kb (or lesser the pKb) of a base. stronger is the base.
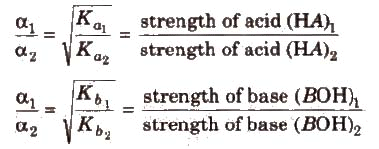
Levelling Effect
The adds like HClO4 H2SO4, HNO3 etc. react with water almost completely to form H3O+ions. Therefore, all the strong acids in aqueous solutionsappear equally strong and their relative strengths in aqueous solution cannot be compared. Since H3O+ is the strongest acid in water. the strength of above acids come down to the level of H3O+ strength in water. Similarly.strong bases like NaOH. KOH. Ba(OH)2 come down to the strength of OH– ion in water.
This is called levling effect.
Lewis Concept of Acids and Bases
Lewis acid is a chemical substance which can accept a pair of electrons,
e.g.,
- Molecules with incomplete octet of central atom like AlCl3 ,BeCl2, MgCl2, etc.
- Simple cations like Ag+, Na+, etc.
- Molecules in which the central atom has vacant d-orbital, e.g.,SF4, SnC14 PF3 etc.
Lewis base is a chemical substance which can donate a pair of electrons. e.g.,
- Neutral molecules containing lone pairs like NH3, RNH2, ROH etc.
- Negatively charged species like CN, Cl. OH, etc.
- In coordination complexes, the ligands act as Lewis base.
Limitations of Lewis Concept
- It does not explain the behaviour of protonic acids such as HCl, H4SO4, HNO3 etc.
- It does not predict the magnitude of relative strength of acids and bases.
All Bronsted-Lowry’s acids are Lewis acids while acids need not be Bronsted-Lowry’s acids.
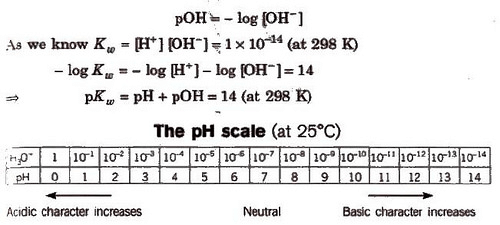
The Ionization Constant of Water Ionic product is the product of the concentration of hydronium ions and hydroxyl ion in pure water, which remains constant at a particular temperature. It is symbolized by Kw. At 298 K, ionic product of water (KW) is given as KW: = [H3O+] [OH–] = 1 x 10-14 mol2L–.
The value of Kw increases with increase in temperature.
The pH Scale
pH is defined as the negative logarithm of hydrogen ion concentration.
pH = – log [H+] and [H+] = lO-pH
Total [H+] or [OH–] in a mixture of two strong acids or bases = (ΣNV/ΣV)
Similarly, negative logarithm of hydroxyl ion concentration is pOH.
pH value of an acid having H+ concentration less than 10-7, is always in between 6 and 7. For 10-8 N HCl solution. it is 6.958.Similarly, for 10-8 NaOH solution, the pH is 7.04 (because basic solutions always have pH 77.)
pH of solution is accurately measured by pH meter or emf method or roughly by pH paper or indicator paper.
(PH can be zero in 1 N Hel solution or it can be negative for more. concentrated solution like 2N, 3N, lON, etc,
pH range for some important substances are :
Gastric juice = 1 – 3
Vinegar = 2.4 – 3.4
Tears – 7.4
Human urine – 4.8 – 8.4
Blood plasma – 7.3 – 7.4
Boil water – 6.5625
Dissociation Constant of Weak
Acid and Weak Base
Let us consider the dissociation of weak acid (HA) as
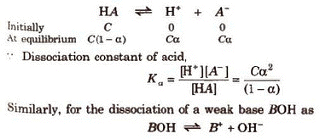
Dissociation constant for polyprotic acids and bases. For a tribasic acid,
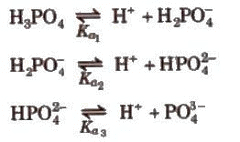
The overall dissociation constant (K) is given as
K = K1 x K2 x K3
where, K1 x K2 x K3

Similarly, for a dibasic acid like H2CO3
pH = pka1 + pka2/2)
Buffer Solution
Solution which resists the change in its pH value by addition of a small amount of acid or a base, is called buffer solution.
- Acidic buffer They have pH value < 7, e.g., CH3COOH/CH3COONa, bone acid/borax.
- Basic buffer They have pH value> 7 e.g., NH4OH/NH34Cl
Buffer system present in blood is H2CO3 + NaHCO3.
Henderson-Hesselbalch Equation
Equation used to calculate the pH of a buffer solution.
(i) For acidic buffer,
pH = pKa + log[salt/acid]
(i) pOH = pKb + log[salt/acid]
and pH = 14 – pOH
Here, pKa = -log Ka, pKb = -log Kb and Ka and Kb are dissociation constants of acid and base.
[salt), [acid] and [base) represent molar concentrations of salt, acid and base respectively.
If addition of a strong acid or base changes the pH of a buffer by/unit, the buffer solution is assumed to destroyed, i.e.,
New pH = pKo ± 1
This means the ratio.
[salt]/[acid) or [salt]/[base] = 10 or (1/10)
Buffer Capacity
It is defined as the number of moles of acid or base added in 1 L of solution Lochange the pH by unity.
Buffer capacity (φ) = (no. of moles of acids or base added to 1 L of buffer/change in pH)
Salts
These are the product of reaction between an acid and a base.
This reaction is called neutralisation reaction.
For Acidic Buffer mixture( Henderson- Hasselbalch equation)
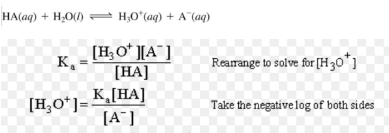
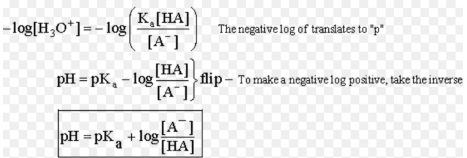
For Basic Buffer mixture ( Henderson- Hasselbalch equation)
pOH = pKb + log [salt] / [Base]
pH + pOH =14
pOH = 14 – pH
pKa + pKb = 14
pKb=14 – pKa
14 – pH = 14 – pKa + log [salt) / [base]
pH = pKa – log [salt] / [base]
pH = pKa + log [base] / [salt]
where Ka is the ionization constant of the conjugate acid of the base.
For ex: In the buffer NH4OH + NH4Cl , NH4+ is the conjugate acid of the base, NH3and Ka represents the ionization constant of the reaction:
NH4+ (aq) + H2O (l) NH3 (aq) + H+ (aq)
pH = pKa + log [NH3] / [NH4+]
pH = pKa + log [Base] / [conjugate acid]
Buffer Capacity
It is defined as the number of moles of an acid or a base required to be added to one litre of the buffer solution so as to change its pH by one unit.
Buffer Capacity = No. of moles of the acid or base added to 1 litre of the buffer / change in pH
Buffer capacity of a buffer is maximum when the concentration of the weak acid and its salt or weak base and its salt are equal i.e. when pH = pKa or pOH = pKb
Importance of Buffer Solution
1) In biological processes
The pH of our blood is maintained constant inspite of various acid and base producing reactions going on in our body.The buffer action is due to the presence of carbonic acid , bicarbonate ion and carbon dioxide in the blood.
2) In industrial processes
The use of buffers is an important part of many industrial processes, e.g.,
in electroplating, in the manufacture of leather, dyes, photographic materials.
3) In analytical chemistry
1) in the removal of acid radicals such as phosphate, oxalate and borate which interfere in the precipitation of radicals of group 3.
2) in complexometric titration
3) to calibrate the pH metres
4)In bacteriological research, culture media are generally buffered to maintain pH required for the growth of the bacteria being studied.
Types of Salts
(a) Normal salts These are obtained by complete neutralisation of an acid with a base, e.g., NaCI, K2SO4, etc.
(b) Acidic salts These are formed by incomplete neutralisation of polybasic acids. e.g., NaHCO3, Na2SO4 etc.
(c) Basic salts These are formed by incomplete neutralization of polyacidic base, e.g., Mg(OH)Cl, Bi(OH)2Cl, etc.
(d) Double salts These are formed by the combination of two simple salts and exist only in solid state, e.g., Mohr salt or ferrous ammonium sulphate (FeSO4.(NH4)2SO4.6H2O], alum, etc.
(e) Complex salts These are formed by the combination of simple salts or molecular compounds. These are stable in solid state well as in solutions.
The properties of their solutions are different from the properties of substances from which they have been constituted.
(f) Mixed salts These salts furnish more than one cation or more than one anion when dissolved in water, e.g., Ca(OCl)Cl, NaKSO4, etc.
Conjugate Acid-Base Pair(CABP) :-
In an acid-base reaction
Acid → H+ conjugate base
Base + H+ → conjugate acid.
e.g.
HCl(aq) + NH3(aq) NH4 (aq) + Cl-(aq)
Note:- A CABP is different from each other only by single proton.
e.g.
HSO-4 is the conjugate base of H2SO4 but SO2-4 is not.
Relative strength of Acids/Bases :-
Any Species and its conjugate species are opposite of each other in terms of strength. e.g.
e.g.
Strength order of acids.
HClO4 > H2SO4 > HCl > CH3COOH
strength order of conjugate bases
ClO4- < HSO4- < Cl- < CH3COO-
An ionic Equilibrium exists between the unionised electroyte molecules and the ions that result from ionisation
Types of keq
1. Self ionization of water
2. Acid dissociation constant
3. Base dissociation constant
4. Salt hydrolysis
5. Sparingly soluble salt
Factors affecting the degree of ionisation and Bronsted and Lowry Concept of Acids and Bases
Factors affecting the degree of ionization:
(a) Temperature - With the rise in temperature, the degree of dissociation of an electrolyte in solution increased. Thus, Degree of dissociation α Temperature
(b) Dilution : On the increasing of dilution, the degree of dissociation increases. But at infinite dilution, their is no effect on the degree of dissociation.
(c) Concentration of the solution :
Degree of dissociation ∝ 1/conc. of the sol. ∝ 1/amount of solute in given vol. ∝ Amount of solvent
(d) Nature of Solvent : Higher the dielectric constant of a solvent, more is its dissociation power or ionising power. Thus ,
Degree of ionisation or dissociation of an electrolyte ∝ dielectric constant of solvent.
Dielectric constant : The dielectric constant of solvent is a measure of its tendency to weaken the forces of attraction between oppositely charged ions of the given electrolyte or the force of attraction applied by solvent molecules on solute molecule is defined as Dielectric constant of solvent.
Note : Water is the most powerful ionizing solvent as its dielectric constant is highest.
(e) Presence of Common Ion : In the presence of a strong electrolyte having common ion, the degree of dissociation of an electrolyte decreases. eg. Ionisation of CH3COOH is suppressed in the presence of HCl due to common H ions.
(f) Nature of Electrolyte : At constant temperature, electrolytes ionize to a different extent in their solutions of same concentration.
Bronsted and lowry concept of acids & bases :
Postulates :-
(1) Acid - Proton (H+ ) donor
(2) Base - Proton (H+ ) acceptor
e.g.
HCl(aq) + H2O(l) H3O+ (aq) + Cl-(aq)
Acid Base ;
HCl(aq) + NH3(aq) NH4+ (aq) + Cl-(aq)
Acid Base;
HCl(aq) + CH3COOH(aq) CH3COOH+2(aq)
Acid Base Cl-(aq);
Note :- Here CH3COOH has a less tendency to donate H than HCl, therefore CH3COOH acts as a weak base.
Ionization of Acid & Bases
The process in which neutral molecules get splits up into charged ions when exposed in a solution is referred to as the ionization of a compound. According to the Arrhenius theory, the acids are the compounds that dissociate in the aqueous medium in order to generate the hydrogen ions, H+ in the aqueous medium.
Ionization of a Compound
While the bases are those compounds that furnish the hydroxyl ions, OH– in the aqueous medium. The degree of ionization of the acids and bases helps determine its strength. On the basis of different acidic and basic compounds, the degree of ionization may differ.
Ionization of Acids
The degree of Ionisation refers to the strength of an acid or a base. A strong acid is said to completely ionize in water whereas a weak acid is said to only ionise partially. As there are different degrees of ionization of acids, there are also different levels of weakness for which there is a simple quantitative way to express.
Since the ionization of a weak acid is an equilibrium, the chemical equation and an equilibrium constant expression can be stated as:
HA (aq) + H2O ( l ) ⇌ H3O+ (aq) + A–
Ka = [ H3O+] [A–] / [HA]
Equilibrium Constant for ionisation of an acid defines its Acid Ionisation Constant (Ka). However, the stronger the acid, the larger will be the acid ionisation constant (Ka). This means that a strong acid is a better proton donor. As a result of the concentration of the product in the numerator of the Ka, the stronger the acid the larger is the acid ionisation constant (Ka).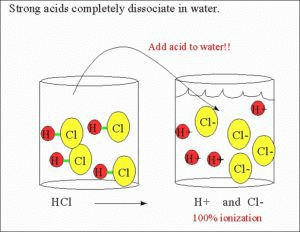
Ionization of Bases
Some bases like lithium hydroxide or sodium hydroxide get completely dissociated into their ions in an aqueous medium which is referred to as strong bases. Therefore, the ionisation of these bases yields hydrochloric ions, as (OH–). A similar expression for the bases is:
A + H2O ⇋ OH– + HA+
Kb = [OH–] [HA+] / [A]
The base ionisation constant i.e Kb refers to the equilibrium constant for the ionisation of a base. Therefore, we can say that a strong base implies a good proton acceptor while a strong acid implies a good proton donor. The dissociation of weak acids or weak bases in water is:
CH3COOH + H2O ⇔ CH3COO‾ + H3O+
NH3 + H2O ⇔ NH4+ (aq) + OH‾(aq)
Solved Examples
Q. A 0.500 M solution of formic acid has a pH value of 2.04. Determine the Ka for formic acid.
Solution: Initial [HCOOH] = 0.500 M and pH = 2.04. Unknown, Ka = ?
[H+] = 10-PH = 10-2.04 = 9.12 x 10-3 M
Now substituting into the Ka expression gives: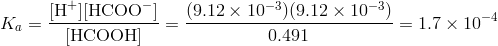
The value of Ka is consistent with that of a weak acid. Now, following the same steps, we find the value of Kb of the base. If 0.750 M solution of the weak base ethylamine (C2H5 NH2) has a pH of 12.31.
C2H5 NH2 + H2O ⇄ C2H5 NH3+ + OH-
The pOH is 14 – 12.31 = 1.69. The [OH−] is found from 10-1.69 = 2.04 × 10-2 M.
The ICE table shall be then as shown below: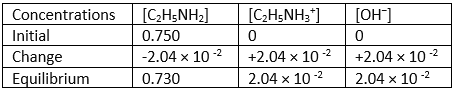
Substituting the value of Kb it yields the Kb for Ethylamine.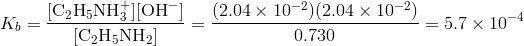
Dissociation Constant and Ionic Product of Water
Pure water is poor conductor of electricity.
Water is a weak electrolyte i.e. it is ionized to a very small extent as:
H2O ⇌ H+ + OH‾
H2O + H2O ⇌ H3O+ + OH‾
This ionization is called self ionization of water.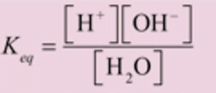
where Keq is the dissociation constant of water.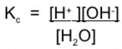
As water is only slightly ionised [H2O] is much bigger then [H+] or [OH-]
∴ we can regard[H2O] as constant
∴ Kc[H2O] = [H+][OH-]
As Kc is aconstant and [H2O] is a constant we can replace both woth a new constant called Kw the ionic product of water
∴ Kw = [H+][OH-]
Kw is the ionic product of water.
Ionic product of water may be defined as the product of the molar concentration of H+ ions and OH‾ ions.
As H+ ions in water exist as H3O+ ions, therefore, ionic product may also be defined as the product of molar concentration of H3O+ ions and OH‾ ions, i.e.
Kw = [H+][OH-]
OR
Kw = [H3O+][OH-]
Ionic product of water is constant only at constant temperature.
Kw at 298 K
Kw = 1.00 × 10-14
Dissociation or ionization of constant of water is different from ionic product of water.
Keq = Kw / 55.55
Kw = Keq × 55.55
Keq = 10-14 / 55.55 = 1.8 × 10-16
In pure water
[H+] = [OH-] = 1 x 10-7mol/L
Kw = [H+][OH-]
= (1 x 10-7)(1 x 10-7)
= 1 x 10-14
Effect of temperature on Kw
The ionic product of water increases with increase of temperature.
With increase of temperature, the degree of ionization of water increases. More of water molecule dissociate into H+ ions and OH‾ ions.The concentration of H+ and OH‾ ions increases and hence the ionic product also increases.
H3O+ ion and OH‾ ion concentration in aqueous solution of acid and bases Pure water
[H+] = [OH-] = 1 x 10-7mol/L
If some acid is added to pure water, then ( H3O+) > 10-7M
[OH-] = Kw / [H+]
If some base is added to pure water, then [OH-] = 10-7 M
[H3O+] = Kw / [OH-]
The increase or decrease of the H3O+ ion concentration in an aqueous solution of an acid or a base may be explained qualitatively on the basis of Le Chatelier’s principle.
2H2O ⇌ H3O+ + OH‾
If some acid is added to pure water, H3O+ ion concentration increases, therefore the equilibrium shifts in the backward direction. Thus OH‾ ion concentration decreases.
If a base added, OH‾ concentration increases. Again the equilibrium shifts in the backward direction and hence the H3O+ ion concentration decreases.
pH Scale and Acidity
The pH scale is a logarithmic scale that is used to measure the acidity or the basicity of a substance. The possible values on the pH scale range from 0 to 14. The term ‘pH’ is an abbreviation of ‘potential for hydrogen’ or ‘power of hydrogen’. Acidic substances have pH values ranging from 1 to 7 (1 being the most acidic point on the pH scale) and alkaline or basic substances have pH values ranging from 7 to 14. A perfectly neutral substance would have a pH of exactly 7.
The pH of a substance can be expressed as the negative logarithm (with base 10) of the hydrogen ion concentration in that substance. Similarly, the pOH of a substance is the negative logarithm of the hydroxide ion concentration in the substance. These quantities can be expressed via the following formulae:
- pH = -log[H+]
- pOH = -log[OH–]
It is important to note that the pH scale is a logarithmic scale. Therefore, an increase in the pH value of a solution by one will be accompanied by a tenfold increase in the hydrogen ion concentration and therefore, a tenfold increase in acidity. Elaborating with the help of an example, a solution with a pH of 3 will have ten times the acidity of a solution with a pH of 4 and a hundred times the acidity of a solution with a pH of 5.
Introduction to pH Scale
Acid solutions have protons and basic solutions have hydroxide ions.
Concentrations of the ions are low (negative power of ten). pH scale is a convenient way of expressing these low concentrations in simple numbers between 1 and 14.
pH is the negative logarithm to the base ten of hydrogen ion concentration in moles per litre.
pH = – log [H+]
p(OH) is the negative logarithm to the base ten of hydroxide ion concentration in moles per litre.
p(OH) = – log [OH–]
In aqueous solutions, pH + p(OH) = 14.
pH scale is based on neutral water, where [H+] = [OH–] = 10-7
For a neutral solution pH = = – log [H+] = – log [10-7] = +7
pH of strong acid decreases with a limit of 1 and pH of a base increases up to 14
Generally, acids and bases will have a pH between.
But negative and greater than14 pH values are also possible.
Limitations of pH Scale
- pH values does not reflect directly the relative strength of acid or bases.
A solution of pH = 1 has a hydrogen ion concentration 100 times that of a solution of pH = 3 (not three times). A 4 x 10-5 N HCI is twice concentrated of a 2 x 10-5 N HCI solution, but the pH values of these solutions are 4.40 and 4.70 (not double). - pH value is zero for 1N solution of strong acid. Concentration of 2 N, 3 N, 10 N, etc. gives negative pH values..
- A solution of an acid having very low concentration, say 10-8 N, shows a pH 8and hence should be basic, but actual pH value is less than 7.
What are Polybasic Acids?
Acids capable of yielding mare than one hydronium ion per molecule are called polybasic acids, the dibasic, tribasic etc indicating the number of replaceable hydrogen.
Taking the example of a few acids such as sulphuric acid, phosphoric acid, we can see that they contain more than one ionizable ion per molecule. Such acids are termed as polybasic acids.
We use so many acids and bases every day, such as vinegar or acetic acid in the kitchen, boric acid for laundry, baking soda for the purpose of cooking, washing soda for cleaning etc. Many of the acids, that we do not consume in the household are used in the laboratories, which includes acids such as HCl, H2SO4 etc. and bases such as NaOH, KOH etc.
Some of these acids and bases have a single hydronium ion or a hydroxyl ion to shed, but most of them have multiple ions. In this section, we will learn about acids and bases which contain more than one ionizable ion per molecule.
Ionization of Polybasic Acids
Let us consider the following ionization reaction of a typical polybasic acid.
H2X(aq) ↔ H+(aq) + HX-(aq)
HX-(aq) ↔ H+(aq) + X-2(aq)
This is an example of dissociation of a dibasic acid into its constituent ions.
The equilibrium constant for the above reaction can be given as,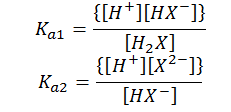
Here, Ka1 and Ka2 are the first and the second ionization constants of the acid H2X. Similarly, for a tribasic acid we have three ionization constants Ka1, Ka2 and Ka3 and so on.
Poly Acidic Bases
The formation of acid salts may be elucidated by the following considerations. Most of the inorganic acids combine with bases in such a manner that 1 atom of the acid is united with 1 atom of a metallic oxide, so that they may be termed monobasic acids.
Certain acids, however, exist of which an instance has already presented itself in the pyrophoosphoric, 1 atom of which possesses the power of combining with 2 atoms of base such acids are hence termed basic. Numerous acids of this class are found among those obtained from the vegetables and the animal kingdoms tartaric acid and malic acid are examples of this kind.
Poly Acidic Base Example
Taking the example of a few bases such as calcium hydroxide, aluminium hydroxide, we can see that they contain more than one ionizable ion per molecule of base. Such acids are termed as poly-acidic bases.
Let us consider the following ionization reaction of a typical poly-acidic base.
M(OH)2(aq) ↔ MOH-(aq) + OH-(aq)
M+OH-(aq) ↔ M+(aq) + (OH)2-(aq)
, this is an example of dissociation of a diacidic base into its constituent ions.
The equilibrium constant for the above reaction can be given as,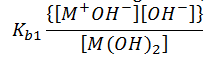
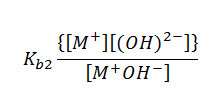
Here, Kb1 and Kb2 are the first and the second ionization constants of the base M(OH)2.
Similarly, for a triacidic base we have three ionization constants Ka1, Ka2 and Ka3 and so on.
What is Acid Strength?
Acid strength is the measure of the ability of the acid to lose its H+ ion.
It depends on several factors which we will discuss in the subsequent sections.
Factors Affecting Acid Strength
- It depends on the strength of the H-A bond. The weaker the bond, the lesser the energy required to break it. Hence, the acid is strong.
- The polarity of the H-A bond affects its acid strength. If the bond is highly polar, the proton tends to leave the molecule more easily, making it a strong acid.
- However, bond strength is more important when we consider and compare acid strengths of elements in the same group of the periodic table, using the above two factors.
- Nevertheless, when we compare the acid strengths of elements in the same row, priority is given to the polarity of the H-A bond.
- The atomic size of A also affects the acid strength. As the atom becomes larger, the bond gets weaker. Consequently, acid strength increases.
pH and the Common-Ion Effect
When the conjugate ion of a buffer solution (solution containing a base and its conjugate acid, or acid and its conjugate base) is added to it, the pH of the buffer solution changes due to the common ion effect.
- An example of such an effect can be observed when acetic acid and sodium acetate are both dissolved in a given solution, generating acetate ions. However, sodium acetate completely dissociates but the acetic acid only partly ionizes. This is because acetic acid is a weak acid whereas sodium acetate is a strong electrolyte.
- As per Le Chatelier’s principle, the new acetate ions put forth by sodium acetate facilitate the suppression of the ionization of acetic acid, thereby shifting the equilibrium to the left. Since the dissociation of acetic acid is reduced, the pH of the solution is increased.
- Therefore, the common ion solution containing acetic acid and sodium acetate will have an increased pH and will, therefore, be less acidic when compared to an acetic acid solution.
Thus, the common ion effect, its effect on the solubility of a salt in a solution, and its effect on the pH of a solution.
Hydrolysis of Salts: Introduction
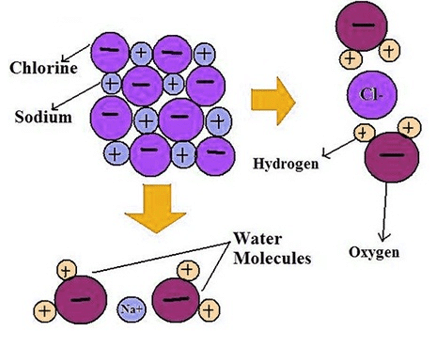 Salt is a compound formed by the neutralization reaction between an acid and a base. They generally ionize in water furnishing cations and anions. The cations or anions formed during ionization of salts either exist as hydrated ions in aqueous solutions or interact with water to regenerate the acids and bases. The process of interaction between cations or anions of salts and water is known as hydrolysis of salts. On the basis of hydrolysis, salts are divided into three categories:
Salt is a compound formed by the neutralization reaction between an acid and a base. They generally ionize in water furnishing cations and anions. The cations or anions formed during ionization of salts either exist as hydrated ions in aqueous solutions or interact with water to regenerate the acids and bases. The process of interaction between cations or anions of salts and water is known as hydrolysis of salts. On the basis of hydrolysis, salts are divided into three categories:- Acidic salts
- Basic salts
- Neutral salts
Let us discuss hydrolysis of salts of the following types:
- Salts of strong acid and strong base: Salts formed by the neutralization of strong acid and strong base are neutral in nature as the bonds in the salt solution will not break apart. They generally get hydrated but do not hydrolyse. Therefore, such salts are generally known as neutral salts. For example: NaCl
- Salts of weak acid and strong base: Salts formed by the neutralization of weak acid and strong base are basic in nature. For example: CH3COONa
CH3COONa (aq) → CH3COO− (aq) + Na+ (aq)
Acetate ion formed undergoes hydrolysis to form acetic acid and OH– ions.
CH3COO−(aq) + H2O ⇋ Ch3COOH(aq) + OH−(aq)
As we know acetic acid is a weak acid, it remains unionized in the solution. This results in an increase in concentration of OH– ions which makes the solution alkaline. pH of the solution is greater than7. - Salts of strong acid and weak base: Salts formed by the neutralization of strong acid and weak base are acidic in nature. For example: NH4Cl
NH4Cl(aq) → Cl−(aq) + NH+4(aq)
Ammonium ion formed undergoes hydrolysis to form ammonium hydroxide and H+ ions.
NH+4(aq) + H2O ⇋ NH4OH(aq) + H+(aq)
As we know ammonium hydroxide is a weak base, it remains unionized in the solution. This results in an increase in concentration of H+ ions which makes the solution acidic. pH of such solutions is less than 7. - Salts of weak acid and weak base: Salts formed by the neutralization of weak acid and weak base are acidic, basic or neutral depending on the nature of acids and bases involved. For example: CH3COONH4. General mechanism for the hydrolysis of ions formed from these salts:
CH3COO− + NH+4 + H2O ⇋ CH3COOH + NH4OH
Degree of hydrolysis in such cases is independent of the concentration of solution and pH of such solutions is given by:
pH = 7 + 1 / 2 (pKa – pKb)
Hence, we can say that the pH of a solution can be less than 7 or greater than 7 depending on the values of pKa and pKb.
|
334 videos|660 docs|300 tests
|
FAQs on Indicators, Acids, Bases & Salts - Chemistry for JEE Main & Advanced
| 1. What are indicators and how are they used in the context of acids and bases? |  |
| 2. Can you provide some examples of common indicators used in acid-base chemistry? |  |
| 3. How does the ionization of acids and bases affect their pH levels? |  |
| 4. What role do acids and bases play in everyday life? |  |
| 5. Can you explain the concept of pH and how it relates to acids and bases? |  |




















