Chemistry Exam > Chemistry Notes > Inorganic Chemistry > Introduction to Bioinorganic: Essentials & Trace Elements of life
Introduction to Bioinorganic: Essentials & Trace Elements of life | Inorganic Chemistry PDF Download
| Table of contents |

|
| Elements in Body |

|
| Geochemical effects on the distribution of metals |

|
| Classification of elements according to their action |

|
| Metal Ions in the Biological System |

|
| Toxic Metals |

|
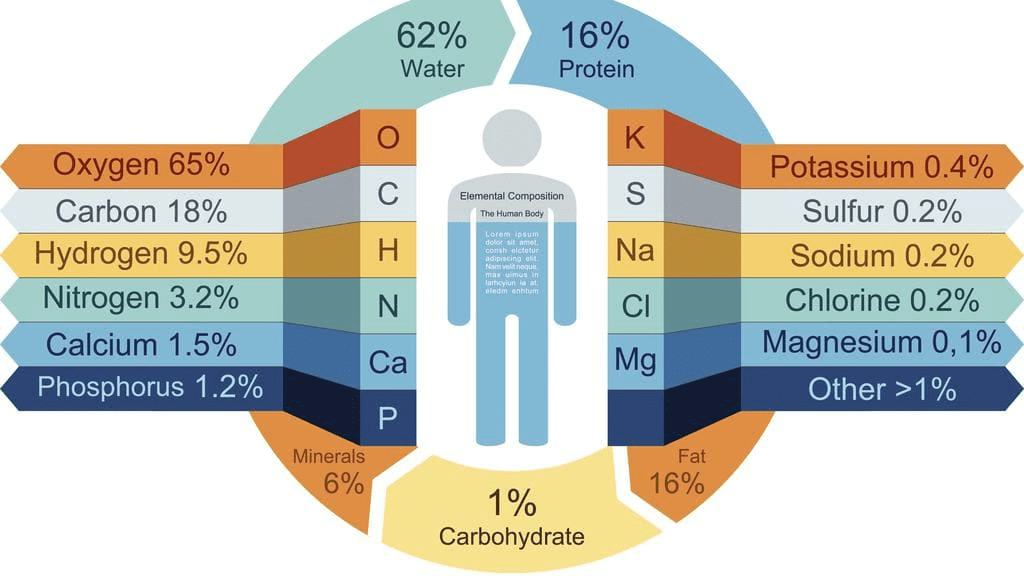
Elements in Body
Consider the content of the elements in the body of an average healthy person (weighing 70 kg). It has been established that out of 70 kg man’s weight
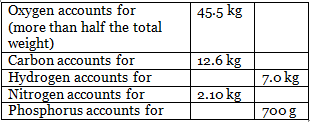
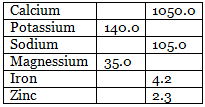
Total Weights of Metals in gms
Geochemical effects on the distribution of metals
All the most essential elements, except Mo are fairly abundant in the earth’s crust.
- Al (8.2%), Si (28.2%), Ti (0.57%) and Zr (0.02%) are although abundant but these are not essential elements.
- Because all these form insoluble oxides at biological pH values and do not form stable complexes with complexing agents of biological significance.
- No common element is toxic at levels normally encountered though almost anything can be harmful at too high levels.
- All the well-known toxic elements, which are currently of much concern in environmental pollution problems, are extremely rare in abundance in the earth's crust.
As (~ 2 х 10PP%), Pb (~ 1.3 х 10PP%), Cd (~ 2 х 10PP%) and Hg (~ 5 х 10PP%).
Classification of elements according to their action
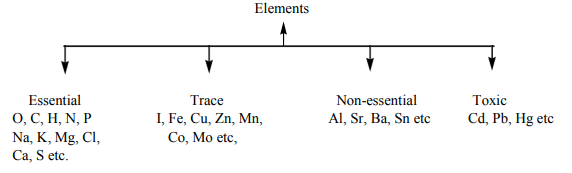
- Essential elements are absolutely essential or necessary for life processes. Trace elements are also necessary for life processes.
- Non-essential elements are not essential. If they are absent other elements may serve the same function.
- Toxic elements disturb the natural functions of the biological system.
Question for Introduction to Bioinorganic: Essentials & Trace Elements of lifeTry yourself:Which is an Essential Element?
View Solution
Metal Ions in the Biological System
Metal ions their excess and deficiency
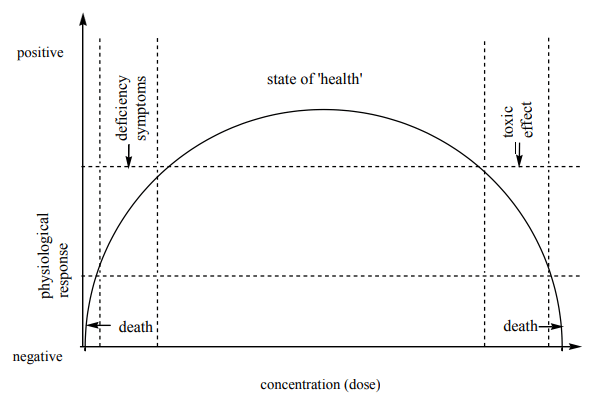
- The concentration of metal ions in a human being’s system is controlled within very fine limits.
- This control is generally exercised by certain biological complexing agents. The deficiency or excess of metal ions causes disorder, which leads to various diseases.
Calcium
- Calcium is a critical element in all animals and man. The primary dietary source of Ca is milk (65-76%), with smaller amounts derived from meat, fish, and eggs (5-10%), and still less from non-dairy foods such as nuts, fruits, beans, etc.
- Dietary deficiency of Ca is not a common problem in nations with high dairy products and protein intake, particularly since normal individuals can regulate intestinal absorption and renal conservation mechanism with great precision.
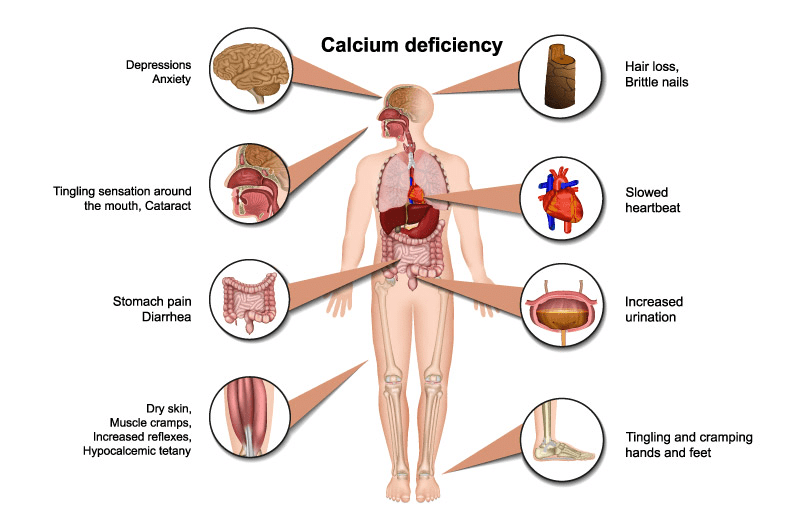
- Hence, human health problems related to the geochemical distribution of Ca, its entry into the human food chain, and its bio-availability are relatively uncommon.
- Exceptions include very poor diets (such as those low in milk and animal proteins or unusual physiologic, other illnesses, such as intestinal malabsorption).
- In case of excess Ca, it comes into the blood as Ca is rejected by cells and its salts are not soluble. So an excess of Calcium leads to the formation of stones, hardening of arteries, and cataracts in the eye.
Magnesium
- Magnesium, an abundant element in the earth’s crust, is vital to both plant and animal life. Chlorophyll pigment in plants is an Mg-porphyrin complex.
- All enzymatic reactions in animals and men that are catalyzed by ATP require Mg as a cofactor. Oxidative phosphorylation, DNA transcription, RNA function, protein synthesis, and critical cell membrane functions are all dependent upon optimal Mg concentrations.
- Dietary sources high in Mg include nuts, seafood, legumes, and vegetables, meat is intermediate in Mg content.
Potassium
- An adult human has approximately 140 g K of which >90% is both intracellular and exchangeable (K is the predominant cation in intracellular water) since muscle contains most of the body’s intracellular water, it also contains most of the K.
- Since K is found in most animal and vegetable foods, dietary deficiency is exceedingly rare except under unusual conditions (such as diets very high in refined sugars, alcoholic individuals deriving most of their calories from low-K alcoholic beverages in the states of starvation, etc.).
Sodium
- Sodium is the predominant extracellular cation in animals and man. An adult human has about 105 g Na, about 24% is located in bone and about 65% in extracellular water.
- Sodium-ion equilibrium is maintained primarily by the kidney, the key organ in water and electrolyte balance. Sodium chloride (salt) is the predominant dietary source.
- Although excessive dietary Cl appears to have no significant ill effect on health, there is much evidence that excessive Na intake results in elevated blood pressure (hypertension) and that reduces Na intake or increased K intake helps to reduce high blood pressure.
Cobalt
- Cobalt is an essential element for humans, but its pathway through the food chain to human beings remain elusive.
- Only a little over 1 mg Co is present in an adult human. In man, dietary Co deficiency is only likely among strict vegetarians or when the intrinsic factor from the stomach that facilitates decreased as in pernicious anaemia.
Zinc
- An adult has about 1.5-3.0 g Zn with the largest amounts being in the liver and bone. There is evidence that Zn concentrations in blood and several tissues vary considerably in response to many stimuli.
- Zinc appears to be critical in many functions. Human Zn deficiency in an inherited form in infants is termed acrodermatitis enteropathica and is characterized by behavioral disturbances, diarrhoea, hair loss, and severe peri-orificial skin rash, all of which respond with remarkable promptness to Zn administration.
- Similar syndromes have now been reported many times with penicillamine treatment of other disorders, presumably due to chelation of Zn, as well as during total parenteral nutrition when Zn was not added to the nutritional solutions for even as short a time as two weeks.
Molybdenum
- The essentiality of Mo in animals and human beings is assumed from its presence in the metalloenzymes xanthine oxidase and aldehyde oxidase.
- Mo is also part of the enzyme sulfite oxidase, an inherited deficiency of which causes severe neurologic disorders and early death in humans.
- However, no naturally occurring Mo deficiency has ever been documented in animals or men, even though several animal deficiencies have been produced experimentally, particularly by using the Mo antagonist.
- Molybdenum is present in very small quantities. Molybdenum appears to be readily absorbed from the GI tracts and excreted primarily through the kidneys (though human studies are lacking).
- In tissues with higher concentrations, such as bone, liver, and kidney the Mo content can be varied with dietary intake. There is evidence that dietary Mo affects Cu metabolism in animals and men, with higher Mo intakes causing mobilization and excretion of Cu.
- These effects can be elicited in man even with naturally occurring dietary sources of Mo. Since Mo concentrations in grains and vegetables vary enormously (differences up to 500 times) and vary with soil content the possibility of Mo-induced Cu deficiency in man is conceivable, though not reported.
Chromium
- The designation of Cr as an element essential to animals and man is quite recent. Insofar as is known, the major biological function of Cr is an integral part of an organic complex originally isolated from yeast termed “glucose tolerance factor” (GTF).
- This complex apparently includes one Cr (III) ion and two nicotinic acid molecules and may coordinate with three aminoacid molecules, probably glycine, cysteine, and glutamic acid.
- Experimental data indicate that GTF functions in conjunction with insulin, and may in fact aid in binding insulin to sites of action. Other activities apparently include a lowering of serum cholesterol and triglycerides.
- An adult human has about 6 mg Cr. Trivalent Cr is absorbed in the upper gastrointestinal tract, but only in very small amounts (hexavalent Cr is better absorbed, but only trivalent Cr is biologically active as an essential element). Trivalent Cr as GTF is apparently absorbed much better.
- Thus conversion to GTF in the gastrointestinal tract may be important and may vary with age of the individual. Chromium in excess amounts can be quite toxic, dependent upon the chemical species of Cr(III) is much less toxic than the hexavalent form.
- Chromium is a known carcinogen and toxic metal present abundantly in tannery effluents in India. As an estimate, about 80-90% of the tanneries use chromium as a tanning agent.
- Of this quantity, the hides take up only 50-70%, while the rest is discharged as effluent. This rest amounts to nearly 75, 000 tonnes per day. Today, the tannery industry mushrooming in North India has converted the Holy Ganges River into a dumping ground.
- Several analyses reveal high concentrations of chromium even in supposedly “treated” effluents. The residues can be traced even in crops cultivated with water taken from the river.
- The Department of Environment has identified the tannery industry as the 'biggest pollutant' across the country. Electroplating can also release chromic acid spray and air-borne Cr trioxide, both of which can result in direct damage to skin and lungs.
Copper
- Normal adult human has about 100-110 mg Cu, highest concentrations are found liver, kidney, heart, and brain.
- The prototype functional deficiency of Cu in humans is an X-linked inherited disorder called Menke’s syndrome (Kinky or steely hair syndrome).
- Cu insufficiency was first suspected by analogy with abnormal wool in Cu-deficient sheep. The defect appears to be decreased gastrointestinal absorption and /or cellular utilization of Cu.
- The essentiality of Cu is the consequence of its role in metalloenzymes involving several critical biochemical pathways.
- Copper is accumulated in a number of tissues but in particular is found in the liver, brain and kidney which leads to liver and kidney failure and various neurological abnormalities. Death results if the condition is not recognized and treated.
Iron
- The average human adult has about 4-5 g Fe. Of this amount, about 60-70% is present in hemoglobin in red blood cells, 3-5% is in muscle myoglobin, 15% is bound to the Fe storage cellular protein, ferritin, 0.2% occurs as a component of critical respiratory enzymes and 0.004% is bound to the serum transport protein transferrin.
- Iron deficiency causes anemia because red cells of blood containing less hemoglobin than in normal conditions.
- Acute iron poisoning leads to vomiting, pallor, shock, circulatory collapse, and coma. Chronic conditions are also known in which iron is deposited in tissues and organs of the body.
- This condition is known as siderosis.
Toxicity
The mechanism of the toxicity of metals is very complicated. Generally, toxicity of metals may result from one of the following:
- Blocking the essential biological functional groups of biomolecules such as enzymes. Amino acid residues like serine are –OH functional group, cysteine is –SH group, and histidine –N group often constitute the active sites of enzymes. A toxic metal ion may bind with these functional groups and block the activity of the enzyme.
- Displacing the essential metal ions from biomolecules. A biomolecule with a foreign metal ion loses its activity.
- Modifying the active conformation of biomolecules.
Biomolecules are having specific active conformations and if this active conformation is lost due to the coordination of a metal ion, the activity of the biomolecule is lost.
Toxic Metals
Mercury:
- Sources of Mercury pollution are industrial waste, mining (as mercury is a trace component of many minerals), pesticides, coal &lignite (containing about 100 ppb of Hg).
- It is a well-known toxic metal that came to limelight after the incidence of “Minamata disease” in 1953-60 in Japan. One hundred eleven cases of mercury poisoning were reported who had eaten mercury-contaminated fish from Minamata Bay.
- Among them, 45 people died. The sea fish were found to be containing 27-102ppm of Hg in the form of methyl mercury.
- The mercury source was the effluent (Hg containing catalyst wastes) from a vinyl chloride plant (Minamata Chemical Company) releasing into the bay.
- This was followed by a more tragic report of mercury poisoning from Iraq in 1972, where 450 villagers died after eating wheat that had been dusted with mercury-containing pesticides.
- These two tragic events boosted the awareness of mercury as a pollutant, and ultimately resulting in its being studied more extensively than any other trace elements.
- Mercury can be toxic by ingestion or inhalation, but the toxicity depends upon the chemical form. Soluble inorganic mercury salts are highly toxic.
Reason for Toxicity
- Toxicity of mercury is based on the strong affinity for the deprotonated forms of thiol ligands such as Cysteine; therefore, thiols, RSH, with sulfhydryl group, -SH are also called mercaptans (Mercurium captans).
- So Hg (II) binds strongly with the thiol group of proteins and enzymes and this binding changes the conformation of protein about the active site.
- Mercury is a soft acid and –S of –SH group is a soft base so strong interaction between mercury and -SH group can be explained on the basis of stronger soft-soft binding.
Cadmium:
- The source of Cd pollution in urban areas are metallurgical plants, Cd plating, and battery fabricators. Acute Cd poisoning leads to nausea, salivation, vomiting, diarrhea, and abdominal pain.
- Cd deposition tends to be cumulative in the kidney with lower concentrations in the liver. Another characteristic of Cd poisoning is the brittleness of bones.
- Cd occurs in nature in association with Zn minerals. A severe outbreak of chronic Cd poisoning occurred along the Jintsu river of North-West Japan and was known as Itai- Itai or ouch-ouch disease.
- Over 20 year period around 100 people died. In 1961 Cd was found to be the cause. The region was due to an old disused zinc mine and river water which was containing Cd used for irrigation of rice and people died after eating the rice.
Reason for toxicity
- Cd is similar to zinc. Therefore Cd (II) can displace Zn (II) in many zinc enzymes.
- Like Hg (II), Cd (II) also binds strongly with the –SH groups of Cysteine residues of enzymes e.g., Carbonic anhydrase, dipeptidase, carboxypeptidase, etc.
- Cd (II) like other toxic metal ions affects the active conformation of biomolecules due to the strong binding.
Lead:
Sources of pollution
- The battery industry is the largest single user of lead. But leaded petrol accounts for more than 20% of total lead consumed per year and 90% of lead released to the atmosphere is from gasoline exhaust.
- The triethyl lead cation is formed from tetraethyl lead by the dissociation of a carbanion.
- Toxicity; of this organometallic cation results from the permeability of membranes, including the very discrimination blood-brain barrier causing several disorders of the central and peripheral nervous system (cramps, paralysis, loss of coordination).
- One of the characteristic symptoms of lead poisoning is anemia.
Reason for toxicity
- Like Hg (II) and Cd (II) lead inhibits SH-enzyme but less strongly.
- The major biochemical effect of Pb is its interference with heme synthesis by inhibiting several key enzymes involved in the overall process of heme synthesis.
The document Introduction to Bioinorganic: Essentials & Trace Elements of life | Inorganic Chemistry is a part of the Chemistry Course Inorganic Chemistry.
All you need of Chemistry at this link: Chemistry
|
48 videos|92 docs|41 tests
|
FAQs on Introduction to Bioinorganic: Essentials & Trace Elements of life - Inorganic Chemistry
| 1. What are the main elements found in the human body? |  |
Ans. The main elements found in the human body include oxygen, carbon, hydrogen, nitrogen, calcium, and phosphorus. These elements are essential for the structure and function of our body's organs and systems.
| 2. How do geochemical factors influence the distribution of metals in the environment? |  |
Ans. Geochemical factors such as soil composition, pH levels, and presence of other minerals can affect the distribution of metals in the environment. For example, certain types of soil may have a higher affinity for certain metals, leading to their accumulation in specific areas. Additionally, pH levels can influence the solubility of metals, affecting their mobility and availability to living organisms.
| 3. What is the classification of elements based on their action? |  |
Ans. Elements can be classified based on their action as essential, non-essential, or toxic. Essential elements are necessary for normal physiological functions and include elements like iron, zinc, and copper. Non-essential elements are not required for physiological functions but may have beneficial effects in small amounts. Toxic elements, on the other hand, can cause harm to living organisms even at low concentrations, such as lead, mercury, and cadmium.
| 4. How do metal ions play a role in the biological system? |  |
Ans. Metal ions play crucial roles in various biological processes. They are involved in enzyme catalysis, electron transfer reactions, and maintenance of cell structure. For example, iron ions are essential for the transport and storage of oxygen in red blood cells, while calcium ions are involved in muscle contraction and nerve signaling. Metal ions also contribute to the stability and folding of proteins, acting as cofactors for enzymes.
| 5. What are the toxic effects of metals on living organisms? |  |
Ans. Metals can have toxic effects on living organisms, depending on their concentration and chemical form. Some toxic metals, such as lead and mercury, can accumulate in the body over time and cause damage to organs and tissues. They can disrupt normal cellular functions, interfere with enzyme activity, and induce oxidative stress. Long-term exposure to toxic metals can lead to various health problems, including neurological disorders, kidney damage, and cardiovascular diseases.

|
Explore Courses for Chemistry exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
Related Searches












