Oxides, Hydroxides and Salt of First Row Metals | Inorganic Chemistry PDF Download
Properties of Transition Metals and Their Compounds
We have daily contact with many transition metals. Iron occurs everywhere—from the rings in your spiral notebook and the cutlery in your kitchen to automobiles, ships, buildings, and in the hemoglobin in your blood. Titanium is useful in the manufacture of lightweight, durable products such as bicycle frames, artificial hips, and jewelry. Chromium is useful as a protective plating on plumbing fixtures and automotive detailing.
Transition metals are defined as those elements that have (or readily form) partially filled d orbitals. As shown in Figure 19.1.2 , the d-block elements in groups 3–11 are transition elements. The f-block elements, also called inner transition metals (the lanthanides and actinides), also meet this criterion because the d orbital is partially occupied before the f orbitals. The d orbitals fill with the copper family (group 11); for this reason, the next family (group 12) are technically not transition elements. However, the group 12 elements do display some of the same chemical properties and are commonly included in discussions of transition metals. Some chemists do treat the group 12 elements as transition metals. Figure: Transition metals often form vibrantly colored complexes. The minerals malachite (green), azurite (blue), and proustite (red) are some examples. (credit left: modification of work by James St. John; credit middle: modification of work by Stephanie Clifford; credit right: modification of work by Terry Wallace)
Figure: Transition metals often form vibrantly colored complexes. The minerals malachite (green), azurite (blue), and proustite (red) are some examples. (credit left: modification of work by James St. John; credit middle: modification of work by Stephanie Clifford; credit right: modification of work by Terry Wallace)
The d-block elements are divided into the first transition series (the elements Sc through Cu), the second transition series (the elements Y through Ag), and the third transition series (the element La and the elements Hf through Au). Actinium, Ac, is the first member of the fourth transition series, which also includes Rf through Rg.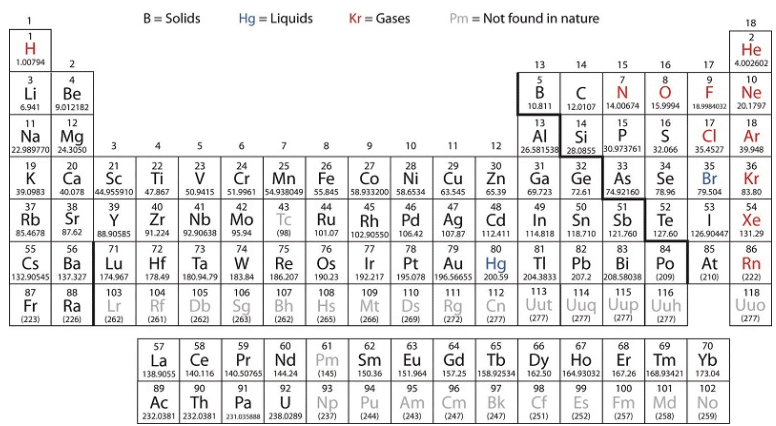 Figure : The transition metals are located in groups 3–11 of the periodic table. The inner transition metals are in the two rows below the body of the table.
Figure : The transition metals are located in groups 3–11 of the periodic table. The inner transition metals are in the two rows below the body of the table.
The f-block elements are the elements Ce through Lu, which constitute the lanthanide series (or lanthanoid series), and the elements Th through Lr, which constitute the actinide series (or actinoid series). Because lanthanum behaves very much like the lanthanide elements, it is considered a lanthanide element, even though its electron configuration makes it the first member of the third transition series. Similarly, the behavior of actinium means it is part of the actinide series, although its electron configuration makes it the first member of the fourth transition series.
Example : Valence Electrons in Transition Metals
Q. Review how to write electron configurations, covered in the chapter on electronic structure and periodic properties of elements. Recall that for the transition and inner transition metals, it is necessary to remove the s electrons before the d or f electrons. Then, for each ion, give the electron configuration:
(a) cerium(III)
(b) lead(II)
(c) Ti2+
(d) Am3+
(e) Pd2+
For the examples that are transition metals, determine to which series they belong.
Ans: For ions, the s-valence electrons are lost prior to the d or f electrons.
(a) Ce3+[Xe]4f1; Ce3+ is an inner transition element in the lanthanide series.
(b) Pb2+[Xe]6s25d104f14; the electrons are lost from the p orbital. This is a main group element.
(c) titanium(II) [Ar]3d2; first transition series
(d) americium(III) [Rn]5f6; actinide
(e) palladium(II) [Kr]4d8; second transition series
Q. Check Your Learning Give an example of an ion from the first transition series with no d electrons.
Ans: V5+ is one possibility. Other examples include Sc3+, Ti4+, Cr6+, and Mn7+.
Properties of the Transition Elements
Transition metals demonstrate a wide range of chemical behaviors. As can be seen from their reduction potentials (Table P1), some transition metals are strong reducing agents, whereas others have very low reactivity. For example, the lanthanides all form stable 3+ aqueous cations. The driving force for such oxidations is similar to that of alkaline earth metals such as Be or Mg, forming Be2+ and Mg2+. On the other hand, materials like platinum and gold have much higher reduction potentials. Their ability to resist oxidation makes them useful materials for constructing circuits and jewelry.
Ions of the lighter d-block elements, such as Cr3+, Fe3+, and Co2+, form colorful hydrated ions that are stable in water. However, ions in the period just below these (Mo3+, Ru3+, and Ir2+) are unstable and react readily with oxygen from the air. The majority of simple, water-stable ions formed by the heavier d-block elements are oxyanions such as MoO2−4 and ReO−4.
Ruthenium, osmium, rhodium, iridium, palladium, and platinum are the platinum metals. With difficulty, they form simple cations that are stable in water, and, unlike the earlier elements in the second and third transition series, they do not form stable oxyanions.
Both the d- and f-block elements react with nonmetals to form binary compounds; heating is often required. These elements react with halogens to form a variety of halides ranging in oxidation state from 1+ to 6+. On heating, oxygen reacts with all of the transition elements except palladium, platinum, silver, and gold. The oxides of these latter metals can be formed using other reactants, but they decompose upon heating. The f-block elements, the elements of group 3, and the elements of the first transition series except copper react with aqueous solutions of acids, forming hydrogen gas and solutions of the corresponding salts.
Transition metals can form compounds with a wide range of oxidation states. Some of the observed oxidation states of the elements of the first transition series.. As we move from left to right across the first transition series, we see that the number of common oxidation states increases at first to a maximum towards the middle of the table, then decreases. The values in the table are typical values; there are other known values, and it is possible to synthesize new additions. For example, in 2014, researchers were successful in synthesizing a new oxidation state of iridium (9+).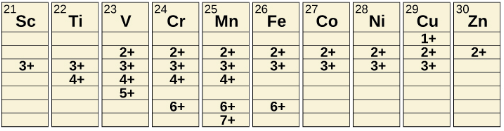 Figure: Transition metals of the first transition series can form compounds with varying oxidation states.
Figure: Transition metals of the first transition series can form compounds with varying oxidation states.
For the elements scandium through manganese (the first half of the first transition series), the highest oxidation state corresponds to the loss of all of the electrons in both the s and d orbitals of their valence shells. The titanium(IV) ion, for example, is formed when the titanium atom loses its two 3d and two 4s electrons. These highest oxidation states are the most stable forms of scandium, titanium, and vanadium. However, it is not possible to continue to remove all of the valence electrons from metals as we continue through the series. Iron is known to form oxidation states from 2+ to 6+, with iron(II) and iron(III) being the most common. Most of the elements of the first transition series form ions with a charge of 2+ or 3+ that are stable in water, although those of the early members of the series can be readily oxidized by air.
The elements of the second and third transition series generally are more stable in higher oxidation states than are the elements of the first series. In general, the atomic radius increases down a group, which leads to the ions of the second and third series being larger than are those in the first series. Removing electrons from orbitals that are located farther from the nucleus is easier than removing electrons close to the nucleus. For example, molybdenum and tungsten, members of group 6, are limited mostly to an oxidation state of 6+ in aqueous solution. Chromium, the lightest member of the group, forms stable Cr3+ ions in water and, in the absence of air, less stable Cr2+ ions. The sulfide with the highest oxidation state for chromium is Cr2S3, which contains the Cr3+ ion. Molybdenum and tungsten form sulfides in which the metals exhibit oxidation states of 4+ and 6+.
Example: Activity of the Transition Metals
Q. Which is the strongest oxidizing agent in acidic solution: dichromate ion, which contains chromium(VI), permanganate ion, which contains manganese(VII), or titanium dioxide, which contains titanium(IV)?
Ans: First, we need to look up the reduction half reactions (Table P1) for each oxide in the specified oxidation state:
Cr2O2−7+14H++6e−⟶2Cr3++7H2O+1.33V
MnO−4+8H++5e−⟶Mn2++H2O+1.51V
TiO2+4H++2e−⟶Ti2++2H2O−0.50V
A larger reduction potential means that it is easier to reduce the reactant. Permanganate, with the largest reduction potential, is the strongest oxidizer under these conditions. Dichromate is next, followed by titanium dioxide as the weakest oxidizing agent (the hardest to reduce) of this set.
Q. Predict what reaction (if any) will occur between HCl and Co(s), and between HBr and Pt(s). You will need to use the standard reduction potentials from (Table P1).
Ans: Co(s)+2HCl⟶H2+CoCl2(aq) ; no reaction because Pt(s) will not be oxidized by H+
Transition Metal Compounds
The bonding in the simple compounds of the transition elements ranges from ionic to covalent. In their lower oxidation states, the transition elements form ionic compounds; in their higher oxidation states, they form covalent compounds or polyatomic ions. The variation in oxidation states exhibited by the transition elements gives these compounds a metal-based, oxidation-reduction chemistry. The chemistry of several classes of compounds containing elements of the transition series follows.
Halides
Anhydrous halides of each of the transition elements can be prepared by the direct reaction of the metal with halogens. For example:
2Fe(s)+3Cl2(g)⟶2FeCl3(s)
Heating a metal halide with additional metal can be used to form a halide of the metal with a lower oxidation state:
Fe(s)+2FeCl3(s)⟶3FeCl2(s)
The stoichiometry of the metal halide that results from the reaction of the metal with a halogen is determined by the relative amounts of metal and halogen and by the strength of the halogen as an oxidizing agent. Generally, fluorine forms fluoride-containing metals in their highest oxidation states. The other halogens may not form analogous compounds.
In general, the preparation of stable water solutions of the halides of the metals of the first transition series is by the addition of a hydrohalic acid to carbonates, hydroxides, oxides, or other compounds that contain basic anions. Sample reactions are:
NiCO3(s)+2HF(aq)⟶NiF2(aq)+H2O(l)+CO2(g)
Co(OH)2(s)+2HBr(aq)⟶CoBr2(aq)+2H2O(l)
Most of the first transition series metals also dissolve in acids, forming a solution of the salt and hydrogen gas. For example:
Cr(s)+2HCl(aq)⟶CrCl2(aq)+H2(g)
The polarity of bonds with transition metals varies based not only upon the electronegativities of the atoms involved but also upon the oxidation state of the transition metal. Remember that bond polarity is a continuous spectrum with electrons being shared evenly (covalent bonds) at one extreme and electrons being transferred completely (ionic bonds) at the other. No bond is ever 100% ionic, and the degree to which the electrons are evenly distributed determines many properties of the compound. Transition metal halides with low oxidation numbers form more ionic bonds. For example, titanium(II) chloride and titanium(III) chloride (TiCl2 and TiCl3) have high melting points that are characteristic of ionic compounds, but titanium(IV) chloride (TiCl4) is a volatile liquid, consistent with having covalent titanium-chlorine bonds. All halides of the heavier d-block elements have significant covalent characteristics.
The covalent behavior of the transition metals with higher oxidation states is exemplified by the reaction of the metal tetrahalides with water. Like covalent silicon tetrachloride, both the titanium and vanadium tetrahalides react with water to give solutions containing the corresponding hydrohalic acids and the metal oxides:
SiCl4(l)+2H2O(l)⟶SiO2(s)+4HCl(aq)
TiCl4(l)+2H2O(l)⟶TiO2(s)+4HCl(aq)
Oxides
As with the halides, the nature of bonding in oxides of the transition elements is determined by the oxidation state of the metal. Oxides with low oxidation states tend to be more ionic, whereas those with higher oxidation states are more covalent. These variations in bonding are because the electronegativities of the elements are not fixed values. The electronegativity of an element increases with increasing oxidation state. Transition metals in low oxidation states have lower electronegativity values than oxygen; therefore, these metal oxides are ionic. Transition metals in very high oxidation states have electronegativity values close to that of oxygen, which leads to these oxides being covalent.
The oxides of the first transition series can be prepared by heating the metals in air. These oxides are Sc2O3, TiO2, V2O5, Cr2O3, Mn3O4, Fe3O4, Co3O4, NiO, and CuO.
Alternatively, these oxides and other oxides (with the metals in different oxidation states) can be produced by heating the corresponding hydroxides, carbonates, or oxalates in an inert atmosphere. Iron(II) oxide can be prepared by heating iron(II) oxalate, and cobalt(II) oxide is produced by heating cobalt(II) hydroxide:
FeC2O4(s)⟶FeO(s)+CO(g)+CO2(g)
Co(OH)2(s)⟶CoO(s)+H2O(g)
With the exception of CrO3 and Mn2O7, transition metal oxides are not soluble in water. They can react with acids and, in a few cases, with bases. Overall, oxides of transition metals with the lowest oxidation states are basic (and react with acids), the intermediate ones are amphoteric, and the highest oxidation states are primarily acidic. Basic metal oxides at a low oxidation state react with aqueous acids to form solutions of salts and water. Examples include the reaction of cobalt(II) oxide accepting protons from nitric acid, and scandium(III) oxide accepting protons from hydrochloric acid:
CoO(s)+2HNO3(aq)⟶Co(NO3)2(aq)+H2O(l)
Sc2O3(s)+6HCl(aq)⟶2ScCl3(aq)+3H2O(l)
The oxides of metals with oxidation states of 4+ are amphoteric, and most are not soluble in either acids or bases. Vanadium(V) oxide, chromium(VI) oxide, and manganese(VII) oxide are acidic. They react with solutions of hydroxides to form salts of the oxyanions VO3−4 , CrO2−4 , and MnO−4 . For example, the complete ionic equation for the reaction of chromium(VI) oxide with a strong base is given by:
CrO3(s)+2Na+(aq)+2OH−(aq)⟶2Na+(aq)+CrO2−4(aq)+H2O(l)
Chromium(VI) oxide and manganese(VII) oxide react with water to form the acids H2CrO4 and HMnO4, respectively.
Hydroxides
When a soluble hydroxide is added to an aqueous solution of a salt of a transition metal of the first transition series, a gelatinous precipitate forms. For example, adding a solution of sodium hydroxide to a solution of cobalt sulfate produces a gelatinous pink or blue precipitate of cobalt(II) hydroxide. The net ionic equation is:
Co2+(aq)+2OH−(aq)⟶Co(OH)2(s)
In this and many other cases, these precipitates are hydroxides containing the transition metal ion, hydroxide ions, and water coordinated to the transition metal. In other cases, the precipitates are hydrated oxides composed of the metal ion, oxide ions, and water of hydration:
4Fe3+(aq)+6OH−(aq)+nH2O(l)⟶2Fe2O3⋅(n+3)H2O(s)
These substances do not contain hydroxide ions. However, both the hydroxides and the hydrated oxides react with acids to form salts and water. When precipitating a metal from solution, it is necessary to avoid an excess of hydroxide ion, as this may lead to complex ion formation as discussed later in this chapter. The precipitated metal hydroxides can be separated for further processing or for waste disposal.
Carbonates
Many of the elements of the first transition series form insoluble carbonates. It is possible to prepare these carbonates by the addition of a soluble carbonate salt to a solution of a transition metal salt. For example, nickel carbonate can be prepared from solutions of nickel nitrate and sodium carbonate according to the following net ionic equation:
Ni2+(aq)+CO2−3⟶NiCO3(s)
The reactions of the transition metal carbonates are similar to those of the active metal carbonates. They react with acids to form metals salts, carbon dioxide, and water. Upon heating, they decompose, forming the transition metal oxides.
Other Salts
In many respects, the chemical behavior of the elements of the first transition series is very similar to that of the main group metals. In particular, the same types of reactions that are used to prepare salts of the main group metals can be used to prepare simple ionic salts of these elements.
A variety of salts can be prepared from metals that are more active than hydrogen by reaction with the corresponding acids: Scandium metal reacts with hydrobromic acid to form a solution of scandium bromide:
2Sc(s)+6HBr(aq)⟶2ScBr3(aq)+3H2(g)
The common compounds that we have just discussed can also be used to prepare salts. The reactions involved include the reactions of oxides, hydroxides, or carbonates with acids. For example:
Ni(OH)2(s)+2H3O+(aq)+2ClO−4(aq)⟶Ni2+(aq)+2ClO−4(aq)+4H2O(l)
Substitution reactions involving soluble salts may be used to prepare insoluble salts. For example:
Ba2+(aq)+2Cl−(aq)+2K+(aq)+CrO2−4(aq)⟶BaCrO4(s)+2K+(aq)+2Cl−(aq)
In our discussion of oxides in this section, we have seen that reactions of the covalent oxides of the transition elements with hydroxides form salts that contain oxyanions of the transition elements.
High Temperature Superconductors
A superconductor is a substance that conducts electricity with no resistance. This lack of resistance means that there is no energy loss during the transmission of electricity. This would lead to a significant reduction in the cost of electricity.
Most currently used, commercial superconducting materials, such as NbTi and Nb3Sn, do not become superconducting until they are cooled below 23 K (−250 °C). This requires the use of liquid helium, which has a boiling temperature of 4 K and is expensive and difficult to handle. The cost of liquid helium has deterred the widespread application of superconductors.
One of the most exciting scientific discoveries of the 1980s was the characterization of compounds that exhibit superconductivity at temperatures above 90 K. (Compared to liquid helium, 90 K is a high temperature.) Typical among the high-temperature superconducting materials are oxides containing yttrium (or one of several rare earth elements), barium, and copper in a 1:2:3 ratio. The formula of the ionic yttrium compound is YBa2Cu3O7.
The new materials become superconducting at temperatures close to 90 K, temperatures that can be reached by cooling with liquid nitrogen (boiling temperature of 77 K). Not only are liquid nitrogen-cooled materials easier to handle, but the cooling costs are also about 1000 times lower than for liquid helium.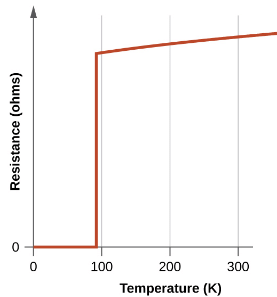
Figure: The resistance of the high-temperature superconductor YBa2Cu3O7 varies with temperature. Note how the resistance falls to zero below 92 K, when the substance becomes superconducting.
Although the brittle, fragile nature of these materials presently hampers their commercial applications, they have tremendous potential that researchers are hard at work improving their processes to help realize. Superconducting transmission lines would carry current for hundreds of miles with no loss of power due to resistance in the wires. This could allow generating stations to be located in areas remote from population centers and near the natural resources necessary for power production. The first project demonstrating the viability of high-temperature superconductor power transmission was established in New York in 2008.
 Figure : (a) This magnetic levitation train (or maglev) uses superconductor technology to move along its tracks. (b) A magnet can be levitated using a dish like this as a superconductor. (credit a: modification of work by Alex Needham; credit b: modification of work by Kevin Jarrett)
Figure : (a) This magnetic levitation train (or maglev) uses superconductor technology to move along its tracks. (b) A magnet can be levitated using a dish like this as a superconductor. (credit a: modification of work by Alex Needham; credit b: modification of work by Kevin Jarrett)
Researchers are also working on using this technology to develop other applications, such as smaller and more powerful microchips. In addition, high-temperature superconductors can be used to generate magnetic fields for applications such as medical devices, magnetic levitation trains, and containment fields for nuclear fusion reactors.
Summary
The transition metals are elements with partially filled d orbitals, located in the d-block of the periodic table. The reactivity of the transition elements varies widely from very active metals such as scandium and iron to almost inert elements, such as the platinum metals. The type of chemistry used in the isolation of the elements from their ores depends upon the concentration of the element in its ore and the difficulty of reducing ions of the elements to the metals. Metals that are more active are more difficult to reduce.
Transition metals exhibit chemical behavior typical of metals. For example, they oxidize in air upon heating and react with elemental halogens to form halides. Those elements that lie above hydrogen in the activity series react with acids, producing salts and hydrogen gas. Oxides, hydroxides, and carbonates of transition metal compounds in low oxidation states are basic. Halides and other salts are generally stable in water, although oxygen must be excluded in some cases. Most transition metals form a variety of stable oxidation states, allowing them to demonstrate a wide range of chemical reactivity.
|
50 videos|92 docs|41 tests
|
FAQs on Oxides, Hydroxides and Salt of First Row Metals - Inorganic Chemistry
| 1. What are transition metals and what are their properties? |  |
| 2. What are some examples of transition metal compounds? |  |
| 3. How do transition metal compounds form oxides and hydroxides? |  |
| 4. What is the significance of transition metal compounds being able to form salts? |  |
| 5. How do transition metal compounds exhibit different colors? |  |





















