NMR Spectroscopy | Physical Chemistry PDF Download
What is NMR Spectroscopy?
NMR Spectroscopy is abbreviated as Nuclear Magnetic Resonance spectroscopy.
Nuclear magnetic resonance (NMR) spectroscopy is the study of molecules by recording the interaction of radiofrequency (Rf) electromagnetic radiations with the nuclei of molecules placed in a strong magnetic field.
Zeeman first observed the strange behaviour of certain nuclei when subjected to a strong magnetic field at the end of the nineteenth century, but the practical use of the so-called “Zeeman effect” was only made in the 1950s when NMR spectrometers became commercially available.
It is a research technique that exploits the magnetic properties of certain atomic nuclei. The NMR spectroscopy determines the physical and chemical properties of atoms or molecules.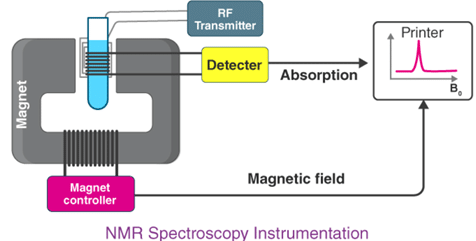
It relies on the phenomenon of nuclear magnetic resonance and provides detailed information about the structure, dynamics, reaction state, and chemical environment of molecules.
Basis of NMR Spectroscopy
Nuclear Magnetic Resonance (NMR) was first detected experimentally at the end of 1945, nearly concurrently with the work groups Felix Bloch, Stanford University and Edward Purcell, Harvard University. The first NMR spectra was first published in the same issue of the Physical Review in January 1946. Bloch and Purcell were jointly awarded the 1952 Nobel Prize in Physics for their research of Nuclear Magnetic Resonance Spectroscopy.
Nuclear magnetic resonance (NMR) spectroscopy is a crucial analytical tool for organic chemists. The research in the organic lab has been significantly improved with the aid of the NMR. Not only can it provide information on the structure of the molecule, it can also determine the content and purity of the sample. Proton (1H) NMR is one of the most widely used NMR methods by organic chemists. The protons present in the molecule will behave differently depending on the surrounding chemical environment, making it possible to elucidate their structure.
NMR Spectroscopy Principle
Many nuclei have spin, and all nuclei are electrically charged, according to the NMR principle. An energy transfer from the base energy to a higher energy level is achievable when an external magnetic field is supplied.
- All nuclei are electrically charged and many have spin.
- Transfer of energy is possible from base energy to higher energy levels when an external magnetic field is applied.
- The transfer of energy occurs at a wavelength that coincides with the radio frequency.
- Also, energy is emitted at the same frequency when the spin comes back to its base level.
- Therefore, by measuring the signal which matches this transfer the processing of the NMR spectrum for the concerned nucleus is yield.
NMR Spectroscopy Working
- Place the sample in a magnetic field.
- Excite the nuclei sample into nuclear magnetic resonance with the help of radio waves to produce NMR signals.
- These NMR signals are detected with sensitive radio receivers.
- The resonance frequency of an atom in a molecule is changed by the intramolecular magnetic field surrounding it.
- This gives details of a molecule’s individual functional groups and its electronic structure.
- Nuclear magnetic resonance spectroscopy is a conclusive method of identifying monomolecular organic compounds.
- This method provides details of the reaction state, structure, chemical environment and dynamics of a molecule.
Chemical Shift in NMR Spectroscopy
A spinning charge generates a magnetic field that results in a magnetic moment proportional to the spin. In the presence of an external magnetic field, two spin states exist; one spin up and one spin down, where one aligns with the magnetic field and the other opposes it.
Chemical shift is characterized as the difference between the resonant frequency of the spinning protons and the signal of the reference molecule. Nuclear magnetic resonance chemical change is one of the most important properties usable for molecular structure determination. There are also different nuclei that can be detected by NMR spectroscopy, 1H (proton), 13C (carbon 13), 15N (nitrogen 15), 19F (fluorine 19), among many more. 1H and 13C are the most widely used. The definition of 1H as it is very descriptive of the spectroscopy of the NMR. Both the nuts have a good charge and are constantly revolving like a cloud. Through mechanics, we learn that a charge in motion produces a magnetic field. In NMR, when we reach the radio frequency (Rf) radiation nucleus, it causes the nucleus and its magnetic field to turn (or it causes the nuclear magnet to pulse, thus the term NMR).
NMR Spectroscopy Instrumentation
This instrument consists of nine major parts. They are discussed below:
- Sample holder – It is a glass tube which is 8.5 cm long and 0.3 cm in diameter.
- Magnetic coils – Magnetic coil generates magnetic field whenever current flows through it
- Permanent magnet – It helps in providing a homogenous magnetic field at 60 – 100 MHZ
- Sweep generator – Modifies the strength of the magnetic field which is already applied.
- Radiofrequency transmitter – It produces a powerful but short pulse of the radio waves.
- Radiofrequency – It helps in detecting receiver radio frequencies.
- RF detector – It helps in determining unabsorbed radio frequencies.
- Recorder – It records the NMR signals which are received by the RF detector.
- Readout system – A computer that records the data.
NMR Spectroscopy Techniques
1. Resonant Frequency It refers to the energy of the absorption, and the intensity of the signal that is proportional to the strength of the magnetic field. NMR active nuclei absorb electromagnetic radiation at a frequency characteristic of the isotope when placed in a magnetic field.
2. Acquisition of Spectra Upon excitation of the sample with a radiofrequency pulse, a nuclear magnetic resonance response is obtained. It is a very weak signal and requires sensitive radio receivers to pick up.
NMR Spectroscopy Applications
- NMR spectroscopy is a Spectroscopy technique used by chemists and biochemists to investigate the properties of organic molecules, although it is applicable to any kind of sample that contains nuclei possessing spin.
- For example, the NMR can quantitatively analyze mixtures containing known compounds. NMR can either be used to match against spectral libraries or to infer the basic structure directly for unknown compounds.
- Once the basic structure is known, NMR can be used to determine molecular conformation in solutions as well as in studying physical properties at the molecular level such as conformational exchange, phase changes, solubility, and diffusion.
|
84 videos|142 docs|67 tests
|
FAQs on NMR Spectroscopy - Physical Chemistry
| 1. What is NMR spectroscopy? |  |
| 2. How does NMR spectroscopy work? |  |
| 3. What are the applications of NMR spectroscopy? |  |
| 4. What are the advantages of NMR spectroscopy? |  |
| 5. Are there any limitations to NMR spectroscopy? |  |
















