Lab Manual: Mixture & Compound | Lab Manuals for Class 9 PDF Download
Objective
To prepare:(i) a mixture
(ii) a compound, using iron filings and sulphur powder and distinguish between these on the basis of :
- appearance i.e., homogeneity and heterogeneity.
- behaviour towards a magnet
- behaviour towards carbon disulphide as a solvent.
- effect of heat.
Theory

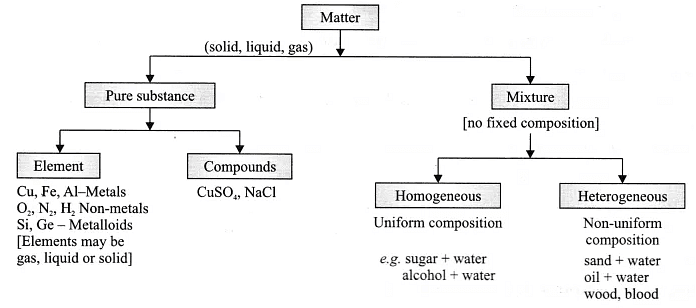
Mixture: When two or more than two substances mix together in any proportion physically and do not show any chemical change, retain their individual properties, then they form a mixture.
Compound: When two or more than two substances combine together chemically in a fixed ratio, such that they can be separated only by chemical means, then a compound is formed.
Differences between Mixture and Compound:
Mixture
Separation of Iron and Sulphur from its Mixture
(i) 
(ii) 
Compound

Separation of Iron and Sulphur from its compound
Materials Required
Test tubes, test tube stand, test tube holder, hard glass test tube, Bunsen burner, tripod stand, wire gauze, magnet, China dish and a watch glass.
Chemicals Required
Iron filings, sulphur powder, carbon disulphide.
Procedure
- Preparation of a mixture of iron and sulphur powder.
Take a pinch of iron filings and two pinch of sulphur powder, mix them thoroughly. The product obtained is mixture of iron and sulphur. Keep it in a watch glass (A). - Preparation of the compound of iron and sulphur.
Take a pinch of iron filing and a pinch of sulphur powder in a hard glass test tube. Hold it in a test tube holder, heat it on the flame till the contents glow. The reaction between sulphur and iron filings is seen in the test tube and iron sulphide is formed. Transfer the compound formed in a watch glass (B).(The mixture of iron filing and sulphur powder can be heated in China dish)
Record your observations in the table.
Observations
Precautions
- Heat the mixture of iron and sulphur in hard glass tube or in a china dish.
- Avoid wasting the chemicals, use very little amount of it.
- Heating activity should be done carefully.
- Carbon disulphide is flammable, keep it away from flame.
|
15 videos|98 docs
|

|
Explore Courses for Class 9 exam
|

|