Class 9 Exam > Class 9 Notes > Extra Documents & Tests for Class 9 > Lab Manual: Osmosis in Raisins
Lab Manual: Osmosis in Raisins | Extra Documents & Tests for Class 9 PDF Download
Objective
To determine the mass percentage of water imbibed by raisins.
Theory
- Imbibition: It is a special type of diffusion in which movement of water takes place due to difference in water molecule concentration between the adsorbant and the imbibant. For e.g., the dry plant part or dry seeds when placed in water increases in size or swells.
Mass percentage of water imbibed by raisins can be calculated as:Mass % of water imbibed=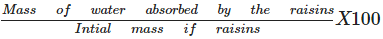
- Osmosis: When the solution of higher water concentration moves into the solution of lower water concentration through a semipermeable membrane, till the concentration on both the sides of the membrane is equal. This process is called osmosis.
- Hypotonic solution: When a cell (dry raisin) is dropped in water, the cell has a less water concentration than the medium in which it is kept. The water molecules will flow into the cell, and it will swell up or even can burst as in case of animal cells. Such a solution is called hypotonic solution. This is Endosmosis.
- Isotonic solution: If the medium has exactly the same water concentration as that of the cell, the water does not move either in or out of the cell, the size of cell remains the same. Such solution is called isotonic solution.
- Hypertonic solution: If the medium has lower concentration of water than the cell, the cell will lose water by osmosis and become smaller in size. Such a solution is called hypertonic solution. This is Exosmosis.
- Plasmolysis: When a cell loses water through osmosis and contracts, the contraction of the contents of the cells is away from the cell wall. This phenomenon is known as plasmolysis.
Materials Required
Five raisins, a beaker, filter paper, weighing balance, watch glass, weight box.
Procedure
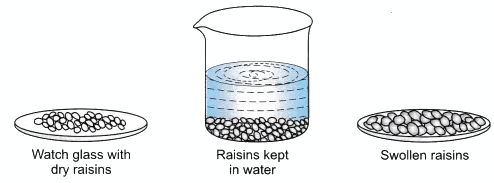
- Take 5 dry raisins and weigh it.
- Place these raisins in a beaker containing water. Allow all the raisins.
- Next day remove all the raisins and place them on filter paper.
- Gently dry the outer surface of raisins by using filter paper.
- Weigh the raisins which are swollen.
Observations
- Weight of raisins taken = 10 g.
- Weight of swollen raisins = 13 g.
Calculations
- Weight of water absorbed by the raisins = (13- 10) = 3 g.
- The percentage of water absorbed by the raisins =
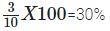
Conclusion
The percentage of water imbibed (absorbed) by raisins is 30 %.
Precautions
- Raisins should be with intact stalks, dry and clean.
- While soaking raisins in water, they should be properly immersed in water.
- Soaking should be for sufficient time.
- Before weighing, gently dry the raisins with the help of filter paper.
The document Lab Manual: Osmosis in Raisins | Extra Documents & Tests for Class 9 is a part of the Class 9 Course Extra Documents & Tests for Class 9.
All you need of Class 9 at this link: Class 9
|
1 videos|228 docs|21 tests
|
FAQs on Lab Manual: Osmosis in Raisins - Extra Documents & Tests for Class 9
| 1. What is osmosis? |  |
Ans. Osmosis is the process by which water molecules move from an area of higher concentration to an area of lower concentration through a semi-permeable membrane.
| 2. How does osmosis occur in raisins? |  |
Ans. In raisins, osmosis occurs when water molecules from the surrounding liquid (usually water or a sugar solution) move into the raisins, which have a higher concentration of solutes. This causes the raisins to swell and become plump.
| 3. What factors can affect the rate of osmosis in raisins? |  |
Ans. The rate of osmosis in raisins can be influenced by several factors such as temperature, concentration of the surrounding liquid, size of the raisins, and duration of the experiment. Higher temperatures and higher concentrations of the surrounding liquid can increase the rate of osmosis.
| 4. How can osmosis in raisins be observed and measured? |  |
Ans. Osmosis in raisins can be observed by placing them in a liquid (such as water or a sugar solution) and monitoring their changes in size and texture over time. The rate of osmosis can be measured by calculating the percentage change in mass or volume of the raisins before and after the experiment.
| 5. What are the practical applications of understanding osmosis in raisins? |  |
Ans. Understanding osmosis in raisins has various practical applications. It helps in understanding processes like food preservation and dehydration. It also provides insights into how cells maintain their water balance and how plants absorb water from the soil. Additionally, it is relevant in the fields of agriculture, biology, and food science.
Related Searches




















