Class 10 Exam > Class 10 Notes > Lab Manuals for Class 10 > Lab Manual: Properties of Acetic Acid
Lab Manual: Properties of Acetic Acid | Lab Manuals for Class 10 PDF Download
Objective
To study the following properties of acetic acid (ethanoic acid):
(ii) Solubility in water
(iii) Effect on litmus
(iv) Reaction with sodium bicarbonate
Materials Required
Test tubes, conical flask, delivery tube, glass rod, acetic acid, blue and red litmus paper or solution, distilled water, sodium hydrogen carbonate (NaHCO3), freshly prepared lime water.
Theory
An organic compound containing the carboxylic group (-COOH) is known as carboxylic acid. Acetic acid is an organic acid with the chemical formula CH3COOH. Its IUPAC name is ethanoic acid.
- Acetic acid is present in vinegar. It can be obtained by wood tar distillation. It is sour in taste. It has a vinegar like smell. Vinegar, commonly called ‘Sirka’, is a dilute (4-5 percent) solution of acetic acid. Acetic acid is highly soluble in water because it gets ionised in aqueous solution and exhibits acidity.
CH3COOH (l)+ H2O (l) → CH3COO–(aq)+ H3O+(aq) - The acidic property of acetic acid is due to the presence of carboxylic group (-COOH), which upon dissociation provide H+(or H3O+) ions. It turns blue litmus to red but does not affect red litmus.
- Acetic acid reacts with sodium bicarbonate and gives brisk effervescence due to the formation of CO2 gas. CO2 turns lime water milky due to the formation of insoluble calcium carbonate and the milkiness disappears if excess of CO2 is passed through the solution.

Procedure
1. Odour- Take 5 ml of acetic acid in a test tube.
- Observe the colour and odour of given acid and note them in observation table.
2. Solubility in Water
- Take 2 ml of water in a test tube and add 1 mL of acetic acid in it and shake it properly.
- Observe the changes occur and write it in the observation table.
3. Effect on Litmus
- Take a blue litmus paper strip and put a drop of acetic acid over it using a clean glass rod as shown in Fig.1.

- Observe the colour change of the litmus paper and write it in the observation table.
- Repeat above steps with red litmus paper.
4. Reaction with Sodium Bicarbonate
- Take another clean test tube and add 2 ml of acetic acid to it.
- Add a pinch of sodium bicarbonate (hydrogen carbonate) to the test tube and shake it properly.
- Hold a burning splinter near the mouth of the test tube.
- On the test tube, put a cork carrying a delivery tube, so that its other end dips in a test tube containing lime water. Allow the gas evolved to pass through lime water (Fig. 2) and write your observation in observation table.

Observation Table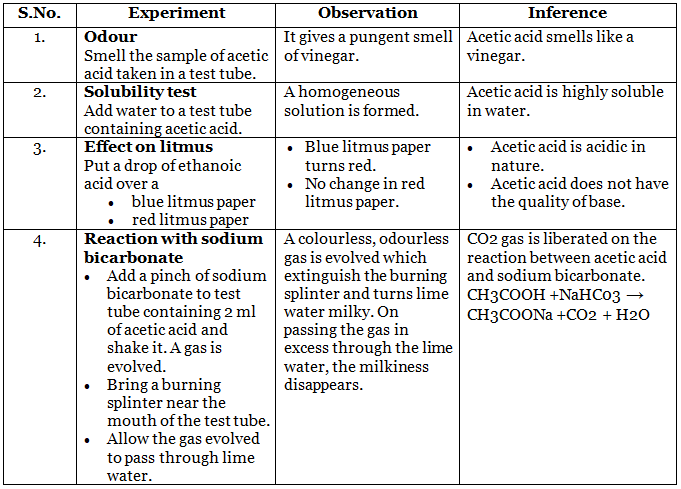
Result
- Acetic acid has a pungent odour of vinegar.
- Acetic acid is completely soluble in water.
- It turns blue litmus paper to red.
- It evolves CO2 gas when reacts with sodium bicarbonate and sodium carbonate.
CH3COOH+ NaHCO3 → CH3COONa+ H2O + CO2
2CH3COOH + Na2CO3 → 2CH3COONa+CO2 + H2O
Lime water turns milky as carbon dioxide evolved above reacts with it to form insoluble calcium carbonate.
Ca(OH)2 + CO2 → CaCO3 + H2O
Precautions
- Ethanoic acid should be handled carefully.
- Do not taste or touch ethanoic acid.
- Do not taste or touch sodium bicarbonate.
- Use small quantities of sodium bicarbonate to control the intensity of CO2 evolved.
- Use clean and dry test tubes.
- Do not inhale vapours of pure acetic acid directly.
- Freshly prepared lime water should be used.
The document Lab Manual: Properties of Acetic Acid | Lab Manuals for Class 10 is a part of the Class 10 Course Lab Manuals for Class 10.
All you need of Class 10 at this link: Class 10

|
Explore Courses for Class 10 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
Related Searches




















