What are Electrophiles and Nucleophiles? | Organic Chemistry PDF Download
Introduction
Before we delve into learning about electrophiles and nucleophiles lets us quickly recall what an attacking reagent is. The species that attacks a substrate molecule or intermediate and forms a product is called attacking reagent.
It is of two types:
- Electrophilic reagent or electrophiles
- Nucleophilic reagent or nucleophiles
Thus, electrophiles and nucleophiles are those types of chemical species that either donate or accept electrons to form a new chemical bond. Meanwhile, the reaction mechanism occurring between electron donors and acceptors are best described by concepts of electrophile and nucleophile. These are the most important concepts in organic chemistry. They were introduced in the year 1933 and replaced the older terms such as cationoid and anionoid.
What is Electrophile?
The word electrophile is made from “electro” derived from electron and “phile” which means loving.
Any molecule, ion or atom that is deficient in electron in some manner can act as an electrophile. In other words, the reagent which attacks the negative of the molecule or loves electrons is called electrophile. They are generally positively charged or are neutral species (electron-deficient molecules) with empty orbitals. Electrophiles can accept a couple of electrons.
Points to Remember
- They are electron deficient and they tend to be attracted towards electrons.
- They are either positively or neutrally charged.
- They attack electron-rich areas such as carbon-carbon double bonds.
- Movement of electrons is affected by the density and the movement is generally from a high-density area to low-density area.
- Favour electrophilic addition and electrophilic substitution reactions.
- Also called Lewis acid because they accept electrons.
- Electrophiles are involved in electrophilic substitution and addition reaction.
Types of Electrophiles
The different types of electrophiles can be classified as:
- Positively Charged Electrophiles: H+, SO3H+, NO+, NO2+, X+, R+ , C6H5N2+, C+2H-OH, CH3 C+ = O
- Neutral Electrophiles: These showcase electron deficiency.
(a) All Lewis acids: BF3, AlCl3, SO3, ZnCl2, BeCl2, FeCl3, SnCl2, CO2, SnCl4.
(b) The neutral atom that accepts electrons from the substrates: > *C = O, R *COCl, R – * Mg – X, *I – Cl, CH3 – *CN, R*–Cl, R*–O,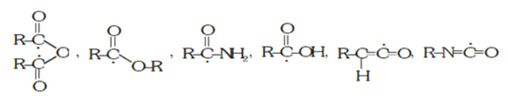 The star (*) indicates the atom that accepts electrons.
The star (*) indicates the atom that accepts electrons.
(c) Free radicals, carbenes and nitrene act as electrophiles.
(d) Species with electrophilic centre: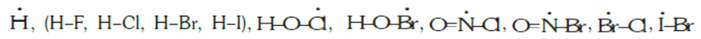
Important information about electrophile
- Hydronium ion carries a positive charge but due to the presence of full vacant orbitals in its outer shell, it does not qualify to be an electrophile.
- In the case of ammonium ion, it does not have vacant orbitals to attract electrons. So ammonium ion not classified as electrophiles.
What is Nucleophile?
A nucleophile is a reactant which gives an electron pair to form a covalent bond. A nucleophile is usually charged negatively or is neutral with a lone couple of donatable electrons. H2O, -OMe or -OtBu are some examples. Overall, the electron-rich species is a nucleophile.
The word nucleophile is made from two words “Nucleo” derived from the nucleus and “phile” which means loving. Species that attacks the positive side of the substrate or loves nucleus are called nucleophiles.
Nucleophiles donate unshared electron pairs, and they act as Lewis bases, according to Lewis ‘notion of acids and bases.
Points to Remember
- They consist of electrons and instead are attracted towards the nucleus. They are either negatively or neutrally charged.
- They are donors of electrons.
- Electrons move from low-density area to high-density area.
- They support nucleophilic addition and nucleophilic substitution reactions.
- Also called as Lewis base.
Types of Nucleophiles
The different types of nucleophiles can be classified as:
- Negatively Charged Nucleophiles:
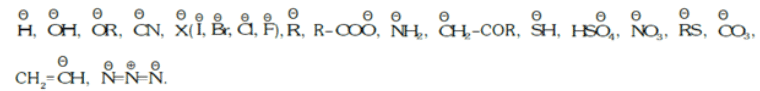
- All Lewis base which contains lone pairs
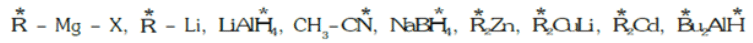
The star (*) indicates the atom which donates electrons to the substrate.- Ambident Nucleophile: Nucleophiles which have two sites of electron-rich centre or in which two or more atoms bear an unshared pair of electrons.
Example: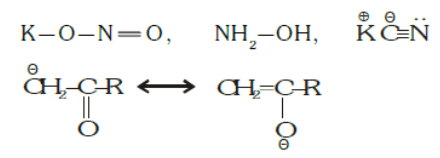
Resonating structures are also ambient nucleophiles. - Amphiphile Nucleophile: Molecule containing multiple bonds between carbon and a more electronegative atom can act both as electrophiles or nucleophiles.
For example:
Difference between Electrophile and Nucleophile
To make you understand how electrophile and nucleophile are different from each other, here are some major differences between them: 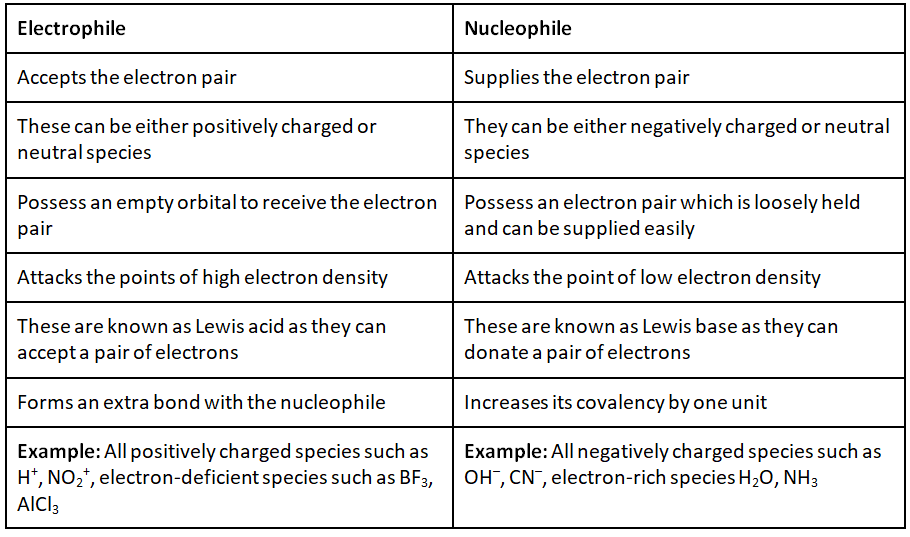
Solved Examples
Example 1: The incorrect statement regarding the nucleophiles is /are?
I. Negatively charged ions are the strongest nucleophiles.
II. The Smaller is the size of the molecule the better is the nucleophile compared to the bigger ions.
III. In polar solvents, bigger atoms are bad nucleophile.
(a) I
(b) II
(c) II and III
(d) III
(e) all statements are correct
Correct Answer is Option (d)
In polar solvents, bigger atoms are good nucleophiles. The bigger the size of the atom, not molecule, the better is the nucleophile (I−> Br−> Cl−> F−). The radius of atoms increases down the group. Nucleophilic strength depends on the strength of the anion. For example, HI is a strong acid, and therefore it has a weak conjugate base.
Example 2: The incorrect statement regarding a nucleophile is/are?
(a) A Fluorine ion is an illustration of a nucleophile.
(b) Weak acids are strong examples of a strong nucleophile.
(c) A strong Arrhenius base is a strong nucleophile.
(d) Nucleophiles tend to give electrons for the formation of a bond.
(e) None of these.
Correct Answer is Option (e)
Nucleophiles are rich in electron species that can form bonding electrophiles (electron-deficient species). Strong bases are strong nucleophiles on the other hand acids are generally weak nucleophiles.
Example 3: Which of the following will be the strongest nucleophile in a nonpolar solution – I, Br, Cl, F?
F
In non-polar solutions, nucleophilic strength increases with increase in electronegativity. Here Fluorine has the highest electronegativity. So it is the strongest nucleophile.
Example 4: The correct order of increasing nucleophilic strength the given compounds will be?
IV, I, III, II
Here two species are negatively charged and the other two are neutral species. Negatively charged species are always more nucleophilic than neutral species. When water and ammonia are compared, the nucleophilic strength of ammonia is more than water because of higher electronegativity of oxygen than nitrogen.
Lesser electronegativity will make electrons more readily available, therefore a stronger nucleophile. Out of Cl− and F−, F− is a stronger nucleophile. This is exceptional to the expected trend. Fluoride ions would be expected to have less nucleophilic strength than chloride ions as the electronegativity of fluorine is more than Chlorine. But the electron density of the chloride ion is more evenly distributed due to its bigger size hence is more stabilized as compared to fluoride ion.
Therefore, the correct order will be IV, I, III, II.
Example 5: Which of the following is the strongest nucleophile?
CH3SeH, CH3OH, CH3SH, CH3TeH
CH3TeH
Here O, Se, S, Te all belong to the same group 6. As we know that size increases down the group therefore the electronegativity decreases. Due to this, the atoms can lose electrons more easily. So there are stronger nucleophiles at the bottom of the group and CH3TeH is the strongest nucleophile.
Example 6: Which of the following compounds would be the best nucleophile?
NH3, CH3S−, CH3SH, H2O, CH3O−
CH3S−
It is the best nucleophile because it has a negative charge (more electron density), and its electrons are held less tightly than those of CH3O− because sulphur is less electronegative than oxygen.
|
44 videos|102 docs|52 tests
|





















