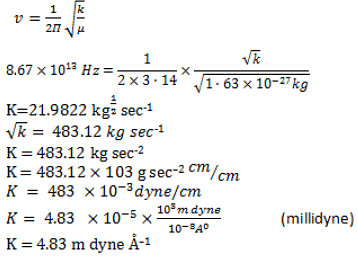Solved Practice Questions on IR Spectroscopy | Organic Chemistry PDF Download
Q.1. What is the correct increasing order of stretching frequencies for C ≡ C, C = C and C — C?
(a) C — C > C = C > C ≡ C
(b) C ≡ C > C = C > C — C
(c) C — C > C = C < C ≡ C
(d) C ≡ C < C — C > C = C
Correct Answer is Option (b)
Since, v ∝ √k , k→ Force constant. Force constant trend is
Triple bond > Double bond > Single bond.
C ≡ C > C = C > C — C.
Q.2. The frequency of vibration of a bond is a function of which factor?
(a) Force constant of the bond
(b) Masses of the atoms involved in bonding
(c) Force constant of the bond and Masses of the atoms
(d) Bond order
Correct Answer is Option (c)
Frequency is directly proportional to force constant and inversely proportional to masses of the atom involved in bonding.
Q.3. Since the nuclei in a polyatomic molecule do not always vibrate in a simple harmonic manner, there arises which of the following situation?
(a) Harmonicity
(b) Anharmonicity in molecular vibrations
(c) Fundamental frequencies
(d) Infrared
Correct Answer is Option (a)
q → displacement and K →Force constant
Since, H–Cl has single bond so having least force constant and respective q is longest among above three. q is larger when the force constant is smaller.
Q.4. In which unit Force constant is not expressed?
(a) Dynes cm-1
(b) dyne Å-1
(c) Nm-1
(d) kp
Correct Answer is Option (d)
All of the above units are correct for force constant except kp, i.e. kilogram force or kilopond, which is the unit of force.
Q.5. The intensity of an absorption band is always proportional to which of the following factor?
(a) Atomic population
(b) Molecular population of the initial state
(c) Molecular population of the final state
(d) Temperature
Correct Answer is Option (b)
The probability of a transition taking place in initial state is the most important factor influencing the intensity of an observed l line. This probability is proportional to the population of the initial state involved in the transition.
Q.6. What is the order of decreasing vibrational frequency for C — Cl, C — Br, C — C, C — O and C — H?
(a) C-H, C-C, C-O, C- Cl, C-Br
(b) C- Cl, C-Br, C-C, C -H, C-O
(c) C-O, C-H, C-Br, C- Cl, C-C
(d) C-Br, C- Cl, C-C, C-O, C-H
Correct Answer is Option (a)
Since,
vibrational frequency inversely proportional to masses of the atom involved in bonding the increasing mass trend for above element is H < C < O < Cl < Br. So, correct order of vibration frequency is C – H > C – C > C – O > C – Cl > C – Br.
Q.7. The vibrations, without a center of symmetry are active in which of the following region?
(a) Infrared but inactive in Raman
(b) Raman but inactive in IR
(c) Raman and IR
(d) Inactive in both Raman and IR
Correct Answer is Option (c)
If a molecule has COS, then its vibrational mode will either IR active or Raman Active.
Q.8. For HCI = 1.63 x 10-27 kg, the observed frequency = 2890 or v = 8.67 x 1013 Hz. What is the force constant K?
(a) 4.83 m dyn Å-1
(b) 8.43 dynes cm-1
(c) 483 μm-1
(d) 4.83 dyn Å-1
Correct Answer is Option (a)
Q.9. On which factors the vibrational stretching frequency of diatomic molecule depend?
(a) Force constant
(b) Atomic population
(c) Temperature
(d) Magnetic field
Correct Answer is Option (a)
The value of vibrating stretching frequency is shifted if the force constant of a bond changes with its electronic structure. Frequency shifts also take place on working with the same substance in different states (solids, liquids and gas). A substance usually absorbs at higher frequency in a gaseous state as compared to liquid and solid states.
Q.10. What is the relation between restoring force, f to the displacement q in Hooke’s law?
(a) f = -kq
(b) f = kq
(c) f = kq2
(d) f = -kq2
Correct Answer is Option (a)
Restoring force f needed to extend or compress a spring by some distance is proportional to that distance. needed to extend or compress a spring by some distance is proportional to that distance.
|
35 videos|92 docs|46 tests
|

|
Explore Courses for Chemistry exam
|

|



 vibrational frequency inversely proportional to masses of the atom involved in bonding the increasing mass trend for above element is H < C < O < Cl < Br. So, correct order of vibration frequency is C – H > C – C > C – O > C – Cl > C – Br.
vibrational frequency inversely proportional to masses of the atom involved in bonding the increasing mass trend for above element is H < C < O < Cl < Br. So, correct order of vibration frequency is C – H > C – C > C – O > C – Cl > C – Br.