Solved Practice Questions on Condensations reactions: Aldol, Claisen, Knoevenagel | Organic Chemistry PDF Download
Q.1. What will be the product ‘B’ in the reaction?
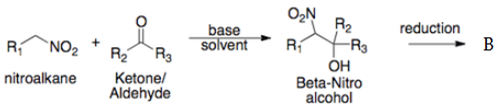
(a) α-nitro alcohol
(b) α-nitro ketone
(c) nitroalkene
(d) β-amino alcohol
Correct Answer is Option (d)
This is a Henry Reaction, it is the combination of a nitroalkane and an aldehyde or ketone in the presence of a base to form β-Nitro alcohols, followed by reduction of the nitro group to yield β-amino alcohols.
Q.2. What will be the product ‘B’ in the reaction?

(a) α-nitro alcohol
(b) α-nitro ketone
(c) nitroalkene
(d) β-amino alcohol
Correct Answer is Option (b)
This is a Henry Reaction, it is the combination of a nitroalkane and an aldehyde or ketone in the presence of a base to form β-Nitro alcohols, followed by oxidation of the secondary alcohol to yield α-nitro ketones.
Q.3. What will be the product for the following reaction?

(a) trans-2,4-pentadienoic acid
(b) cis -2,4-pentadienoic acid
(c) 3-aminobenzoic acid
(d) 2-aminobenzoic acid
Correct Answer is Option (a)
With malonic compounds the reaction product can lose a molecule of carbon dioxide in a subsequent step. In the so-called Doebner modification the base is pyridine. For example, the reaction product of acrolein and malonic acid in pyridine is trans-2,4-Pentadienoic acid with one carboxylic acid group and not two.
Q.4. Which combination of carbonyl compounds gives phenyl vinyl ketone by an aldol condensation?
Find combination of carbonyl compounds gives phenyl vinyl ketone by an aldol condensation
(a) Acetophenone and ketone
(b) Acetophenone and aldehyde
(c) Benzaldehyde and aldehyde
(d) Benzaldehyde and ketone
Correct Answer is Option (a)
Draw the structures of the possible aldol products (3-hydroxy carbonyl compounds) before dehydration from the pairs of reactants shown below, then identify which aldol will lead to phenyl vinyl ketone upon dehydration.
Q.5. Condensation reaction always results in the formation of complex sugar (disaccharide or polysaccharide) and which of the following?
(a) amino acids
(b) lipids
(c) water
(d) maltose
Correct Answer is Option (c)
Condensation reaction always results in the formation of complex sugar (disaccharide or polysaccharide) and water.
Q.6. What will be the product ‘B’ in the reaction?

(a) α-nitro alcohol
(b) α-nitro ketone
(c) nitroalkene
(d) β-amino alcohol
Correct Answer is Option (c)
This is a Henry Reaction, it is the combination of a nitroalkane and an aldehyde or ketone in the presence of a base to form β-Nitro alcohols, followed by dehydration of nitroalkene.
Q.7. What will be the product of the following reaction?
(a) –Nitro alcohol
(b) -nitro alcohol
(c) Nitroalkene
(d) -amino alcohol
Correct Answer is Option (a)
This is a Henry Reaction, it is the combination of a nitroalkane and an aldehyde or ketone in the presence of a base to form β-Nitro alcohols.
Q.8. Which ester will not give a good yield of the Claisen condensation product with NaOEt in EtOH?
(a)
(b)
(c)
(d)
Correct Answer is Option (c)
The Claisen condensation is reversible and it is formation of a stabilized enolate of the product which leads to a high yield at equilibrium. When the product cannot give a stabilized enolate, the yield will be poor.
Q.9. In which condensation an enol or an enolate ion reacts with a carbonyl compound to form a β-hydroxyaldehyde or β-hydroxyketone (an aldol reaction), followed by dehydration to give a conjugated enone happens?
(a) Aldol condensation
(b) Claisen reduction
(c) Henry condensation
(d) Knoevenagel condensation
Correct Answer is Option (a)
Aldol condensation in which condensation an enol or an enolate ion reacts with a carbonyl compound to form a β-hydroxyaldehyde or β-hydroxyketone (an aldol reaction), followed by dehydration to give a conjugated enone happens.
Q.10. Condensation reaction is the reverse of which of the following reaction?
(a) lock and key hypothesis
(b) oxidation
(c) hydrolysis
(d) glycogen formation
Correct Answer is Option (c)
This reaction example is the reverse of hydrolysis, which splits a chemical entity into two parts through action from the polar water molecule, which itself splits into hydroxide and hydrogen ions.
|
35 videos|92 docs|46 tests
|

|
Explore Courses for Chemistry exam
|

|

















