Grade 12 Exam > Grade 12 Notes > Chemistry for Grade 12 > Rutherford's Experiment
Rutherford's Experiment | Chemistry for Grade 12 PDF Download
Introduction
- Understanding the fundamental structure of matter is essential to Physics. Figuring out the size of the nucleus, which is the crux of this article, would not be possible without the Rutherford gold foil experiment. The Rutherford model of the atom was the first correct interpretation of the atom, and it laid the groundwork for Bohr to build his interpretation.
Rutherford Gold Foil Experiment
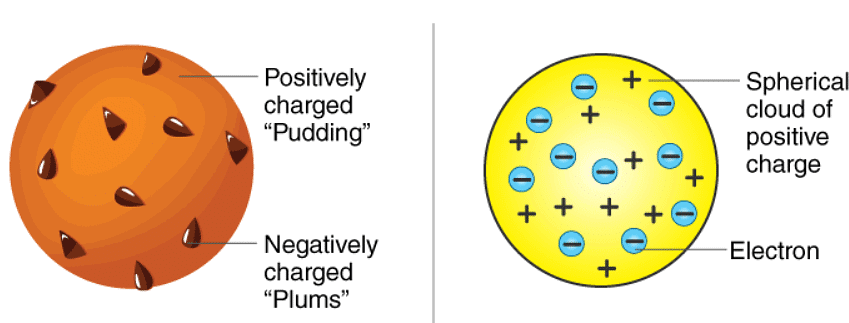
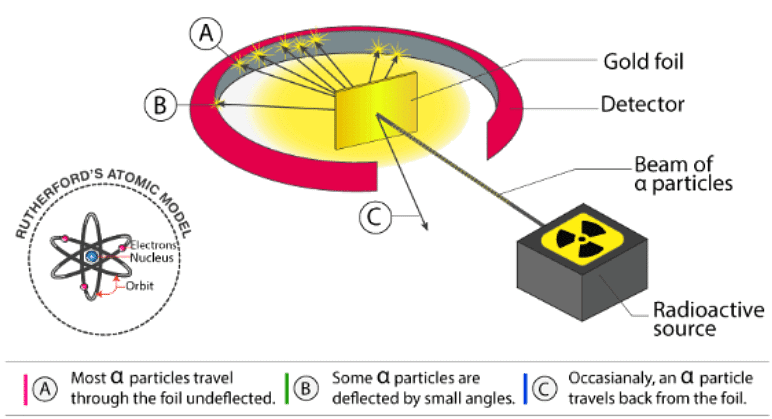
- Before Rutherford’s experiment, the best model of the atom that was known to us was the Thomson or “plum pudding” model. In this model, the atom was believed to consist of a positive material “pudding” with negative “plums” distributed throughout. Later, Rutherford’s alpha-particle scattering experiment changed our perception of the atomic structure. Rutherford directed beams of alpha particles at thin gold foil to test this model and noted how the alpha particles scattered from the foil.

JJ Thompson Plum Pudding Model
- In the experiment, Rutherford showed us that the atom was mainly empty space with the nucleus at the centre and electrons revolving around it. When alpha particles were fired towards the gold foil, Rutherford noticed that 1 in 20000 particles underwent a change in direction of motion of more than 90 degrees. The rest 19999 particles deviated from their trajectory by a very small margin. This led to the conclusion that the atom consisted of an empty space with most of the mass concentrated at the centre in tiny volumes. This volume at the centre was named ‘the nucleus’; Latin for ‘little nut’.

Through this experiment, Rutherford made 3 observations as follows:
- Highly charged alpha particles went straight through the foil undeflected. This would have been the expected result for all of the particles if the plum pudding model was correct.
- Some alpha particles were deflected back through large angles.
- A very small number of alpha particles were deflected backwards! To this, Rutherford remarked, “It was as incredible as if you fired a 15-inch shell at a piece of tissue paper, and it came back at you!”
To explain these observations, a new model of the atom was needed. In the new model, the positive material was considered to be concentrated in a small but massive region called the nucleus. Electrons were considered to be revolving around the nucleus, preventing one atom from trespassing on its neighbour’s space to complete this model.
Size of the Nucleus
- It was possible to obtain the size of the nucleus through Rutherford’s experiment. We can calculate the size of the nucleus, by obtaining the point of closest approach of an alpha particle. By shooting alpha particles of kinetic energy 5.5 MeV, the point of closest approach was estimated to be about 4×10-14m. Since the repulsive force acting here is Coulomb repulsion, there is no contact. This means that the size of the nucleus is smaller than 4×10-14m.
- The sizes of the nuclei of various elements have been accurately measured after conducting many more iterations of the experiment. Having done this, a formula to measure the size of the nucleus was determined.
R = R0A1/3
Where R0 = 1.2 × 10-15m. - From the formula, we can conclude that the volume of the nucleus, which is proportional to R3, is proportional to A (mass number). Another thing to be noticed in the equation is that there is no mention of density in the equation. This is because the density of the nuclei does not vary with elements. The density of the nucleus is approximately 2.3 × 1017 kg.m-3.
The document Rutherford's Experiment | Chemistry for Grade 12 is a part of the Grade 12 Course Chemistry for Grade 12.
All you need of Grade 12 at this link: Grade 12
|
207 videos|373 docs|227 tests
|

|
Explore Courses for Grade 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
Related Searches