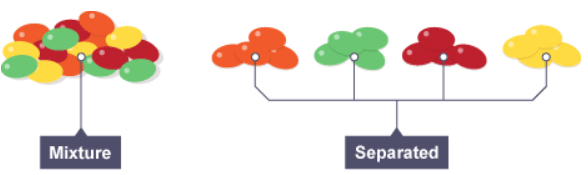
Mixtures | Chemistry for Grade 10 PDF Download
Pure substances and mixtures
The meaning of pure
- The word 'pure' is used in chemistry in a different way from its everyday meaning. For example, shops sell cartons labelled as ‘pure' orange juice. The label means that the contents are just orange juice, with no other substances added. However, the juice is not pure in the chemical sense, because it contains different substances mixed together. In chemistry:
- a pure substance consists only of one element or one compound
- a mixture consists of two or more different substances, not chemically joined together
- The substances in a mixture can be elements, or compounds, or both. Being part of a mixture does not change the chemical properties of the substances that are in it.
Separating mixtures
- Mixtures can be separated by physical processes. These processes do not involve chemical reactions, and no new substances are made.
Filtration and crystallisation
Filtration
- Filtration is used to separate an insoluble solid from a liquid. It is useful for separating sand from a mixture of sand and water, or excess reactant from a reaction mixture.
- Filtration works because the filter paper has tiny holes or pores in it. These are large enough to let small molecules and dissolved ions through, but not the much larger particles of undissolved solid.
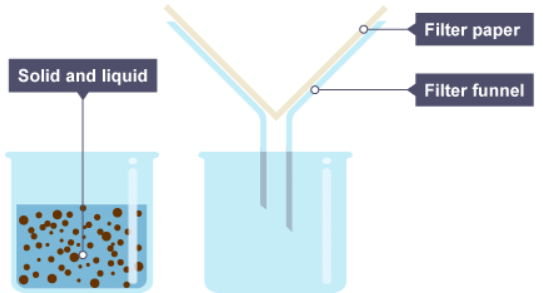
Separating insoluble solids
1. One beaker contains a mixture of solid and liquid, the other contains a funnel with filter paper
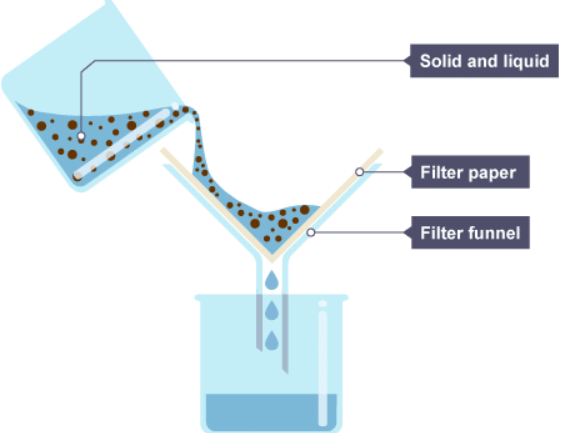
2. The solid and liquid mixture is poured into the filter funnel
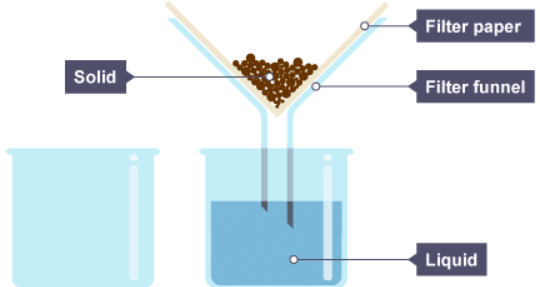
3. The liquid drips through the filter paper but the solid particles are caught in the filter paper
Crystallisation
- Crystallisation is used to produce solid crystals from a solution. When the solution is warmed, some of solvent evaporates leaving crystals behind. For example, crystallisation is used to obtain copper sulfate crystals from copper sulfate solution.
1. A solution is placed in an evaporating basin and heated with a Bunsen burner.
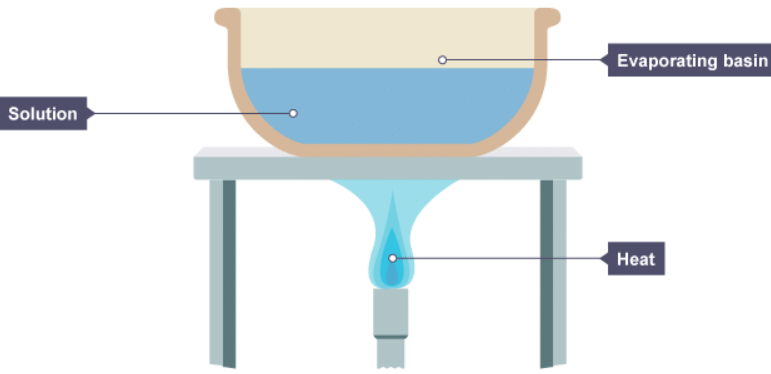
2. The volume of the solution has decreased because some of the water has evaporated. Solid particles begin to form in the basin.
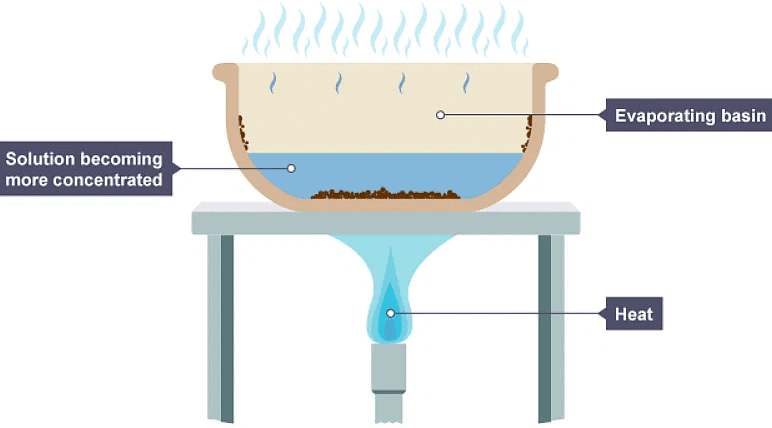
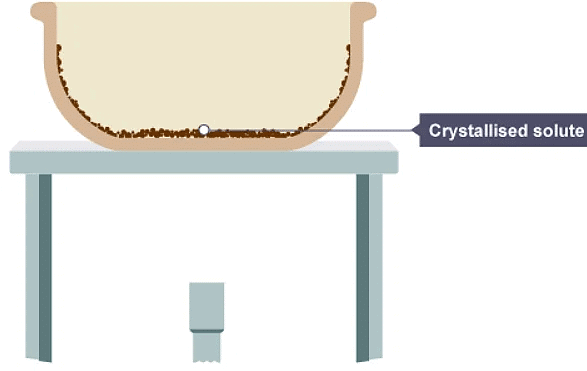
3. All the water has evaporated, leaving solid crystals behind.
To obtain large, regularly shaped crystals from crystallisation:
- put the solution in an evaporating basin
- warm the solution by placing the evaporating basin over a boiling water bath
- stop heating when crystals begin to form around the edge of the basin
After the remaining solution has cooled down, pour the excess liquid away (or filter it). Dry the crystals using a warm oven or by patting them with filter paper.
Distillation
Simple distillation
- Simple distillation is used to separate a solvent from a solution. It is useful for producing pure water from seawater.
- Simple distillation works because the dissolved solute has a much higher boiling point than the solvent. When the solution is heated, solvent vapour leaves the solution. It moves away and is cooled and condensed. The remaining solution becomes more concentrated as the amount of solvent in it decreases.
1. Salty water is heated
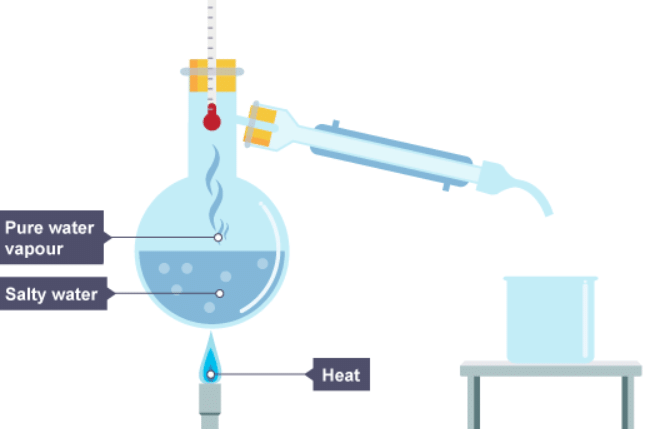
2. The water vapour cools in the condenser and drips into a beaker
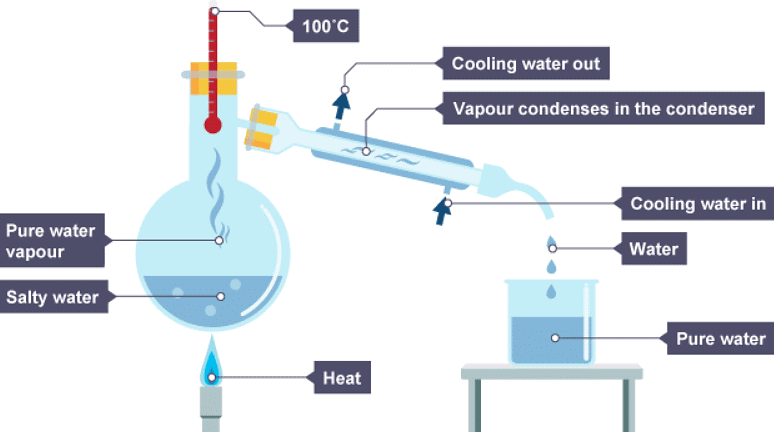
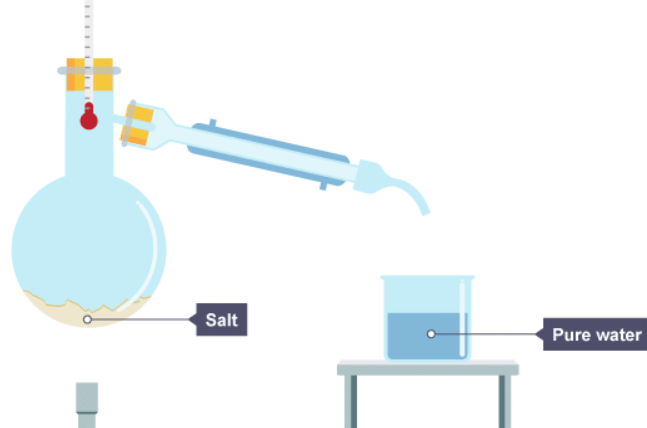
3. The water has condensed and is now in the beaker, the salt stays behind
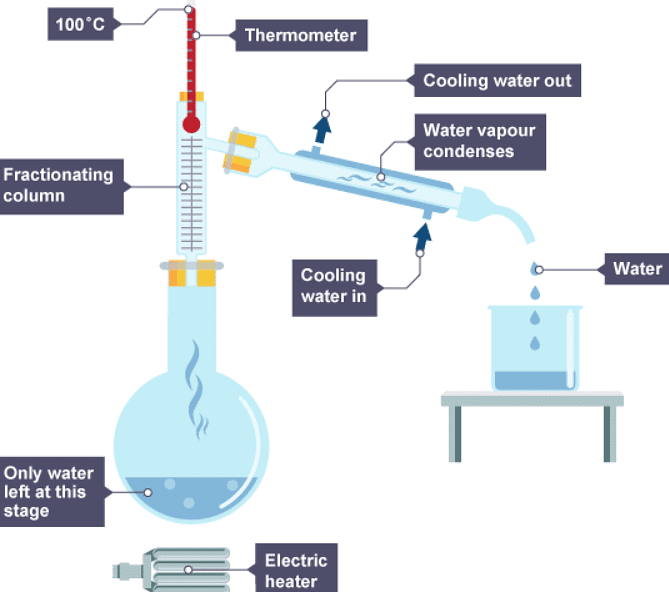
Fractional distillation
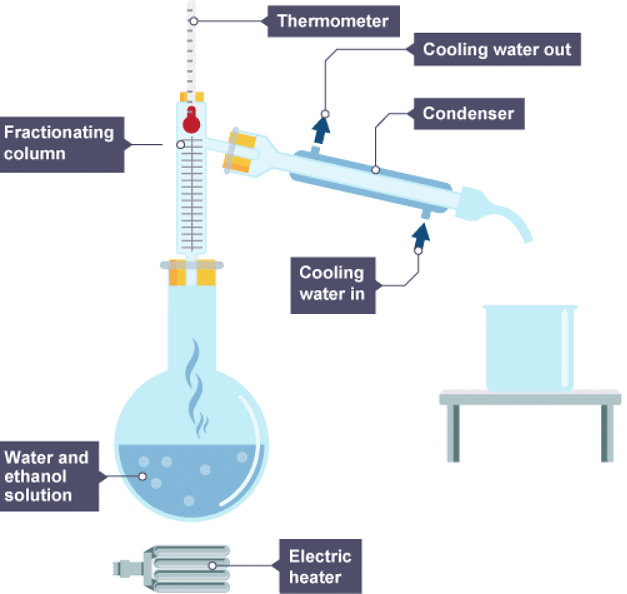
- Fractional distillation is used to separate different liquids from a mixture of liquids. It is useful for separating ethanol from a mixture of ethanol and water, and for separating different fractions from crude oil.
- Fractional distillation works because the different liquids have different boiling points. When the mixture is heated:
- vapours rise through a column which is hot at the bottom, and cooler at the top
- vapours condense when they reach a part of the column that is below the temperature of their boiling point
- each liquid is led away from the column
There are two ways of obtaining different liquids from the column:
- by collecting different liquids from different parts of the column - the substance with the lowest boiling point is collected at the top of the column
- by continuing to heat the mixture to increase the temperatures in the column - the substance with the lowest boiling point is collected first
Distillation process to separate ethanol from water
1. Water and ethanol solution is heated
2. The ethanol evaporates first, cools, then condenses
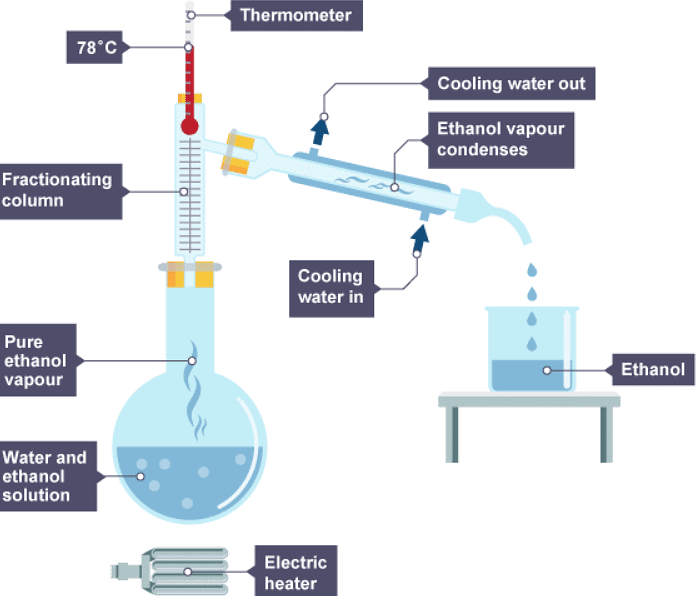
3. The water left evaporates, cools, then condenses
Paper chromatography
- Paper chromatography is used to separate mixtures of soluble substances. These are often coloured substances such as food colourings, inks, dyes or plant pigments.
1. Water and ethanol solution is heated
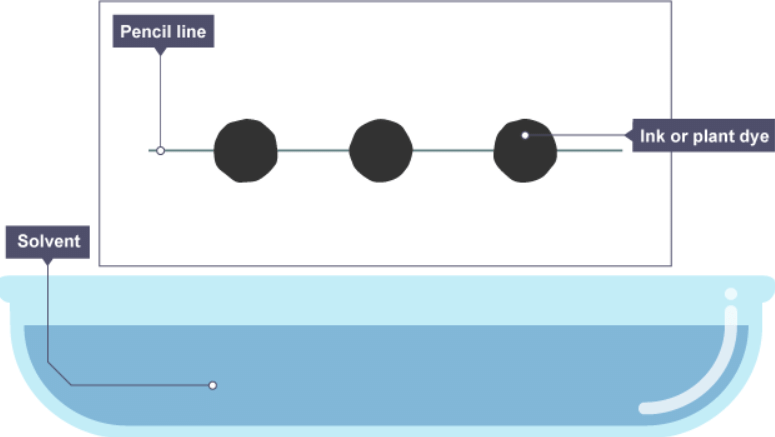
2. As the paper is lowered into the solvent, some of the dye spreads up the paper
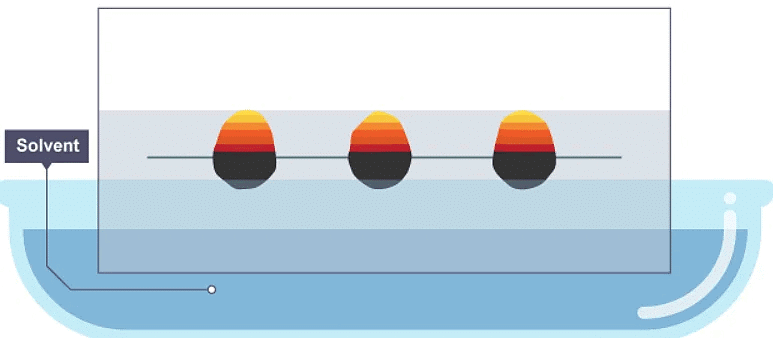
3. The paper has absorbed the solvent, and the dye has spread further up the paper
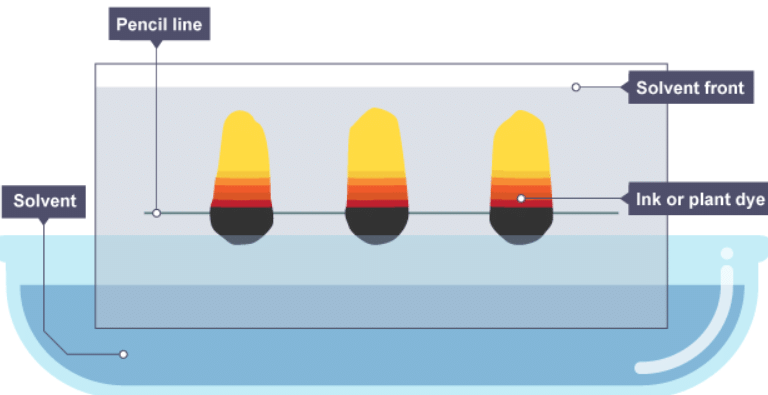
Phases
- Chromatography relies on two different ‘phases’:
- the stationary phase, which in paper chromatography is very uniform, absorbent paper
- the mobile phase is the solvent that moves through the paper, carrying different substances with it
- The different dissolved substances in a mixture are attracted to the two phases in different proportions. This causes them to move at different rates through the paper.
Interpreting a chromatogram
- Separation by chromatography produces a chromatogram. A paper chromatogram can be used to distinguish between pure and impure substances:
- a pure substance produces one spot on the chromatogram
- an impure substance, or mixture, produces two or more spots
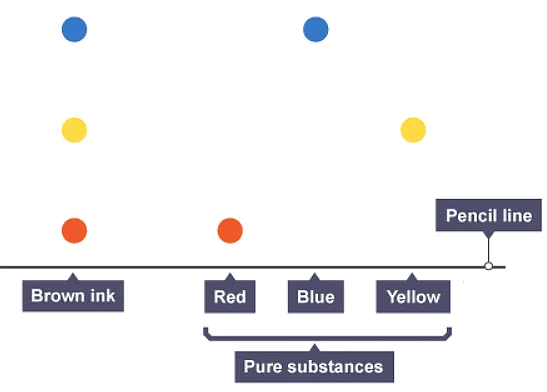
- A paper chromatogram can also be used to identify substances by comparing them with known substances. Two substances are likely to be the same if:
- they produce the same number of spots, and these match in colour
- the spots travel the same distance up the paper
|
75 videos|131 docs|24 tests
|




















