Bonding & Substance Properties | Chemistry for Grade 10 PDF Download
States of Matter
The Physical Forms of Matter
- The three states of matter are solids, liquids and gases
- A substance can usually exist in all three states, dependent on temperature (and pressure)
- State changes occur at the melting point (solid to liquid, liquid to solid) and at the boiling point (liquid to gas and gas to liquid)
- Melting and freezing occur at the melting point
- Boiling and condensing take place at the boiling point
- Individual atoms themselves do not share the same properties as bulk matter
- The three states of matter can be represented by a simple model
- In this model, the particles are represented by small solid spheres
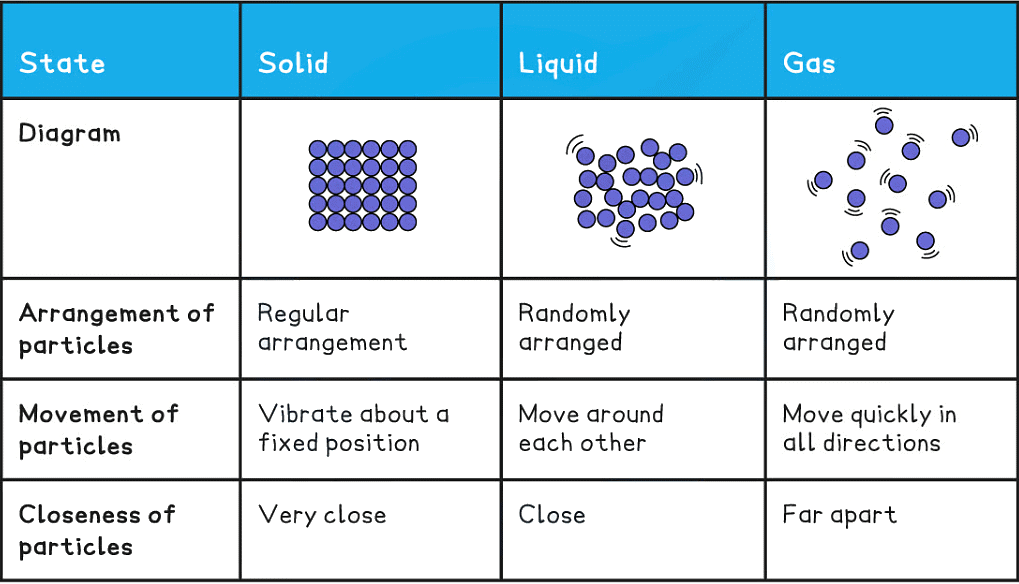
Summary of the Properties of Solids, Liquids and Gases
Energy & State Changes
- The amount of energy needed to change state from solid to liquid and from liquid to gas depends on the strength of the forces between the particles
- The stronger the forces of attraction, the more energy that is needed to overcome them for a state change to occur
- Therefore, the stronger the forces between the particles the higher the melting point and boiling point of the substance
- When matter changes from one state to another due to changes in temperature or pressure, the change is called an interconversion of state
- It is a physical change involving changes in the forces between the particles of the substances, the particles themselves remain the same, as do the chemical properties of the substance
- Physical changes are relatively easy to reverse as no new substance is formed during interconversions of state
- The interconversions have specific terms to describe them:
A Summary of State Changes
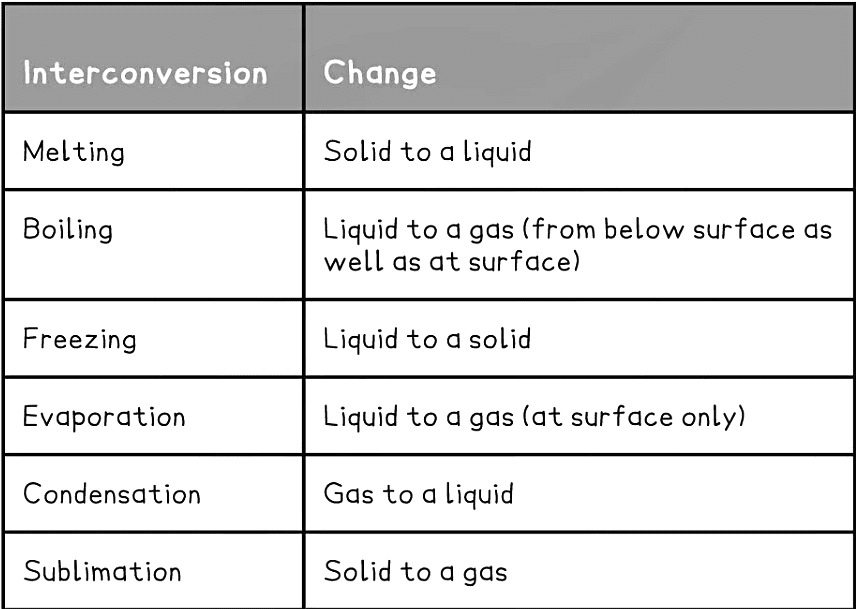
Melting
- Melting is when a solid changes into a liquid
- The process requires heat energy which transforms into kinetic energy, allowing the particles to move
- It occurs at a specific temperature known as the melting point which is unique to each pure solid
Boiling
- Boiling is when a liquid changes into a gas
- This requires heat which causes bubbles of gas to form below the surface of a liquid, allowing for liquid particles to escape from the surface and from within the liquid
- It occurs at a specific temperature known as the boiling point which is unique to each pure liquid
Freezing
- Freezing is when a liquid changes into a solid
- This is the reverse of melting and occurs at exactly the same temperature as melting, hence the melting point and freezing point of a pure substance are the same
- Water for example freezes and melts at 0 ºC
- It requires a significant decrease in temperature (or loss of thermal energy) and occurs at a specific temperature which is unique for each pure substance
Evaporation
- When a liquid changes into a gas
- Evaporation occurs only at the surface of liquids where high energy particles can escape from the liquids surface at low temperatures, below the boiling point of the liquid
- The larger the surface area and the warmer the liquid/surface, the more quickly a liquid can evaporate
- Evaporation occurs over a range of temperatures, but heating will speed up the process as particles need energy to escape from the surface
Condensation
- When a gas changes into a liquid, usually on cooling
- When a gas is cooled its particles lose energy and when they bump into each other, they lack energy to bounce away again, instead grouping together to form a liquid
Sublimation
- When a solid changes directly into a gas
- This happens to only a few solids, such as iodine or solid carbon dioxide
- The reverse reaction also happens and is called desublimation or deposition
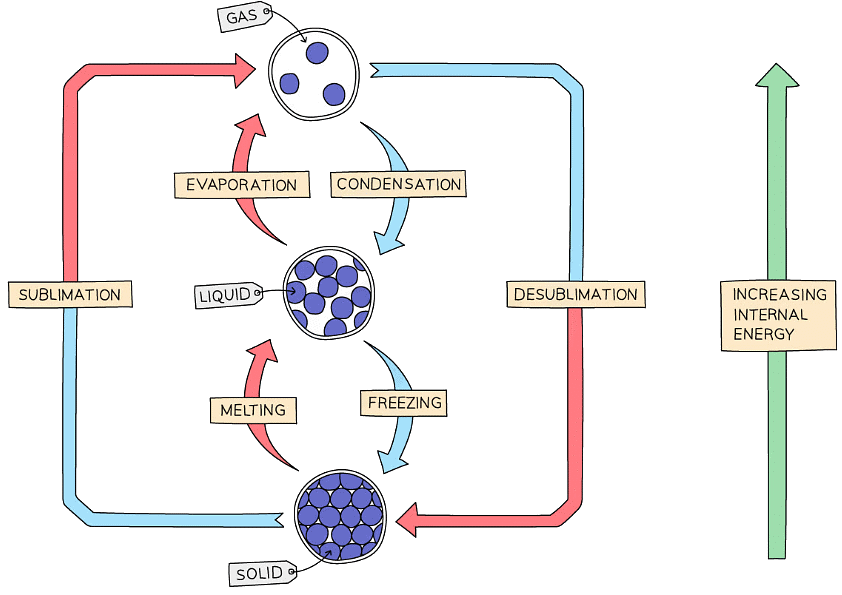
Changing State
Particle Theory & its Limitations
Particle Theory
- Particle theory explains how matter changes state depending on the energy and forces present between the particles in the substance
- The amount of energy needed to change from a solid to a liquid and from a liquid to a gas depends on the relative strength of the forces acting between the particles
- There are many different types of substances which contain different amounts of elements and compounds
- Since each substance contains different particles, then the amount of energy needed to induce a change of state is different for each individual substance
- The stronger the forces between the particles, the higher the energy needed for melting and boiling to occur
- When substances are heated, the particles absorb thermal energy which is converted into kinetic energy
- Heating a solid causes its particles to vibrate more and as the temperature increases, they vibrate so much that the solid expands until the bonds break and the solid melts
- On further heating, the now liquid substance expands more and some particles at the surface gain sufficient energy to overcome the intermolecular forces and evaporate
- When the boiling point is reached, all the particles gain enough energy for the intermolecular forces to break and the molecules to escape as the liquid boils
- While changing state, the temperature of the substance remains the same as the heat energy goes into breaking the bonds between the particles
- This is called latent heat
- The entire process can be summarized in a diagram called a heating and cooling curve
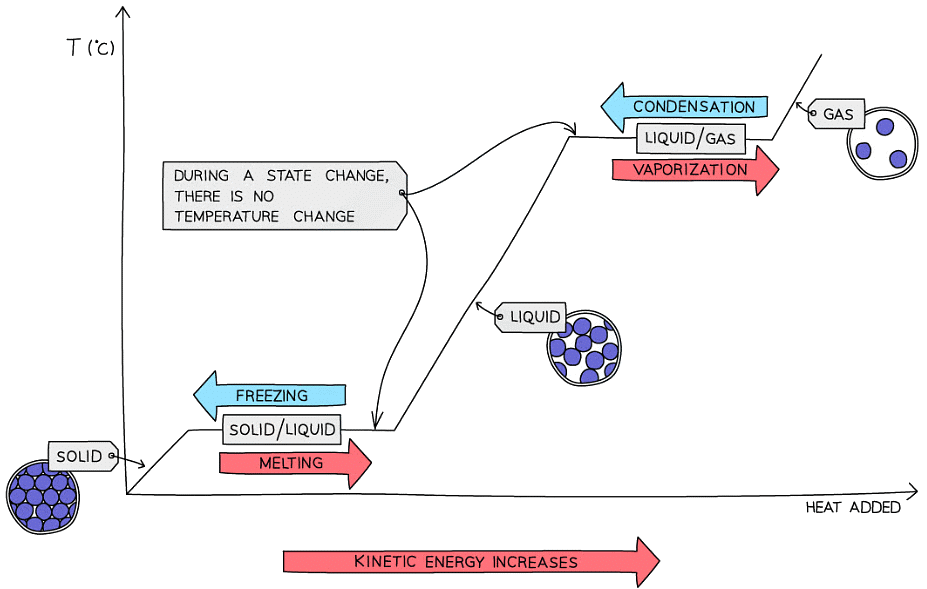
Heating and cooling curve for a pure substance
Limitations of the Particle Theory
- Particle theory considers all particles, irrespective of their state or chemical identity, to be small, solid and inelastic
- It doesn’t consider the difference caused by different particles, such as atoms, ions or molecules or mixtures of all three
- The theory also fails to consider the intermolecular forces that exist between different particles in different substances
Predicting Physical State
- The physical state of a substance under certain conditions can be predicted from a given set of data
- Normally you are given melting and boiling point data for a substance and asked to predict its physical state in specified conditions.
- At temperatures below the melting point:
- The substance will be in the solid state
- At temperatures between the melting point and the boiling point:
- The substance will be in the liquid state
- At temperatures above the boiling point:
- The substance will be in the gas state
Example: The table below indicates melting and boiling point data for four different substances named A, B, C and D.
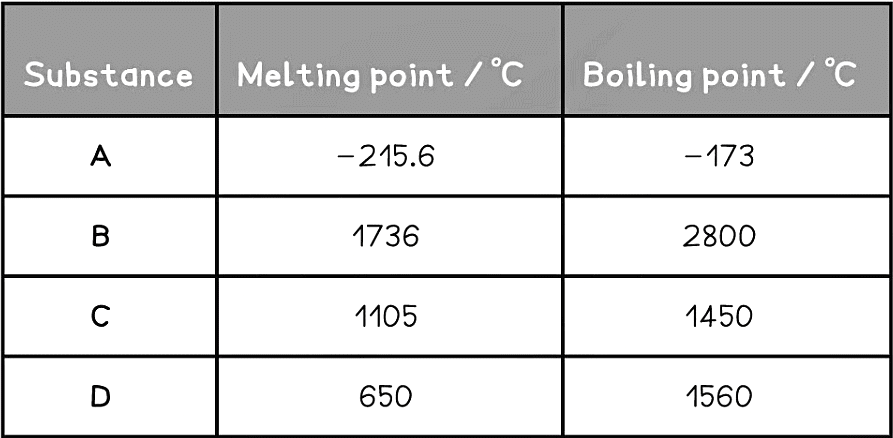
Predict the states of the following substances:
- Substance A at -150 ºC
- Substance B at 50 ºC
- Substance C at 1400 ºC
- Substance D at 400 ºC
- A boils at temperatures above -173 ºC so at -150 ºC A is a gas
- B melts at 1736 ºC so at 50 ºC it is a solid
- C melts at 1105ºC and boils at 1450 ºC so at 1400 ºC it is a liquid
- D melts at 650 ºC so at 400 ºC it is a solid
Solids, Liquids & Gases - A Summary of Properties
Solids
- Strong forces of attraction between particles, particles are packed very closely together in a fixed and regular pattern
- Atoms vibrate in a fixed position but can’t change position or move
- Solids have a fixed volume and shape, and a relatively high density
- Solid particles have only a small amount of energy
Liquids
- There are weaker attractive forces between the particles of a substance in a liquid than in its corresponding solid form
- Particles are close together in an irregular, unfixed form
- Particles can move and slide past each other which is why liquids adopt the shape of the container they are in and also why they are able to flow
- Liquids have a fixed volume but not a fixed shape and have a moderate to high density
- Liquid particles have more energy than those in a solid but less than gaseous particles
Gases
- Particles are in random movement and so there is no defined pattern
- Particles are far apart and move quickly (around 500 m/s) in all directions, they collide with each other and with the sides of the container (this is how pressure is created inside a can of gas)
- No fixed volume, since there is a lot of space between the particles, gases can be compressed into a much smaller volume. Gases have low density
- Gaseous particles have the highest amount of energy
State Symbols
Solid, Liquid, Gas & Aqueous
- State symbols are written after each formula in chemical equations to show which physical state each substance is in
- Brackets are used and they are not usually subscripted although you may come across them written in this way
- Aqueous should remind you of the word 'aqua' and means the substance is dissolved in water
- In other words it is a solution

The four state symbols show the physical state of substances at normal conditions
- Symbol equations should be included when writing chemical equations.
- An example of a reaction with state symbols is the reaction of copper carbonate with hydrochloric acid:
CuCO3 (s) + 2HCl (aq) ⟶ CuCl2 (aq) + CO2 (g) + H2O (l)
Physical Properties of Ionic Compounds
- Ionic compounds are made of charged particles called ions which form a giant lattice structure
- Ionic substances have high melting and boiling points due to the presence of strong electrostatic forces acting between the oppositely charged ions
- These forces act in all directions and a lot of energy is required to overcome them
- The greater the charge on the ions, the stronger the electrostatic forces and the higher the melting point will be
- For example, magnesium oxide consists of Mg2+ and O2- so will have a higher melting point than sodium chloride which contains the ions, Na+ and Cl-
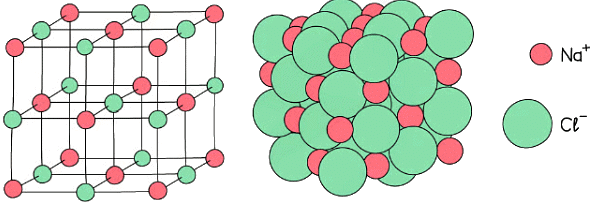
Strong electrostatic forces act in all directions in an ionic solid such as sodium chloride
- Ionic compounds are usually solid at room temperature and are non-volatile
- They are usually water soluble as both ionic compounds and water are polar substances
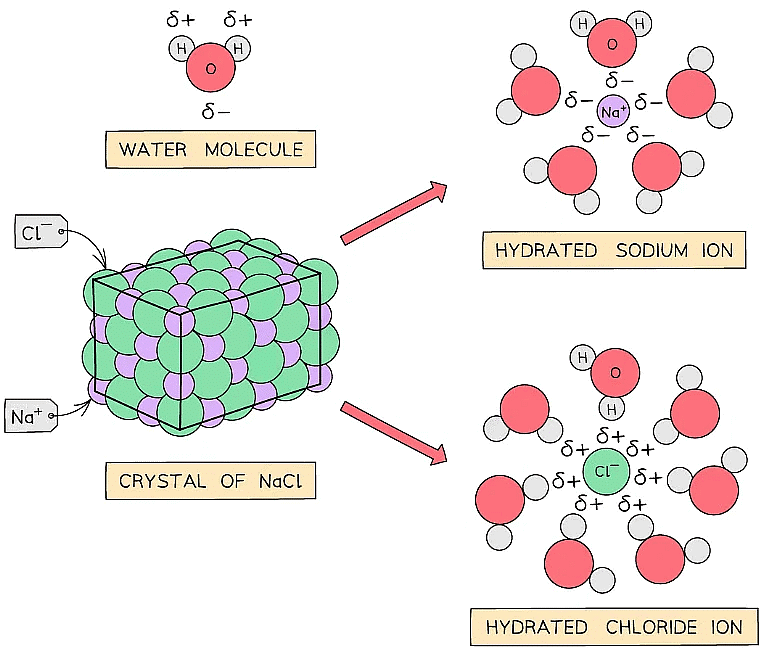
Ionic compounds are soluble in water because the ions are easily hydrated by polar water molecules
Electrical Conductivity: Ionic Compounds
- For electrical current to flow there must be freely moving charged particles such as electrons or ions present
- Ionic compounds can conduct electricity in the molten state or in solution as they have ions that can move and carry charge
- They cannot conduct electricity in the solid state as the ions are in fixed positions within the lattice and are unable to move
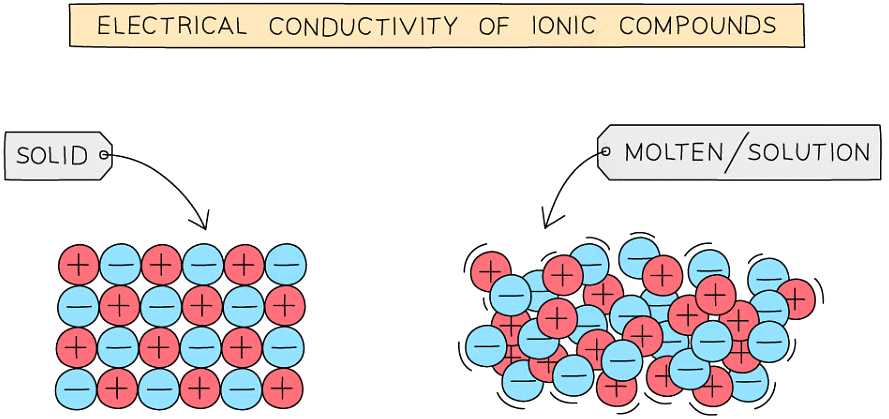 Molten or aqueous particles move and conduct electricity but cannot in solid form
Molten or aqueous particles move and conduct electricity but cannot in solid form
Properties of Small Molecules
Melting & Boiling Points
- Small molecules are compounds made up of molecules that contain just a few atoms covalently bonded together
- They have low melting and boiling points
- This is due to the weak intermolecular forces that require little energy to overcome
- Most covalent compounds are insoluble in water as they tend to be non-polar but can dissolve in organic solvents
- Some do dissolve in water by forming intermolecular attractions with the water molecules
- Examples include sucrose (table sugar C12H22O11) and iodine I2
- As the molecules increase in size, the melting and boiling points generally increase
Electrical Conductivity: Small Molecules
- They are poor conductors of electricity as there are no free ions or electrons to carry the charge.
- Most covalent compounds do not conduct at all in the solid state and are thus insulators
- Common insulators include the plastic coating around household electrical wiring, rubber and wood
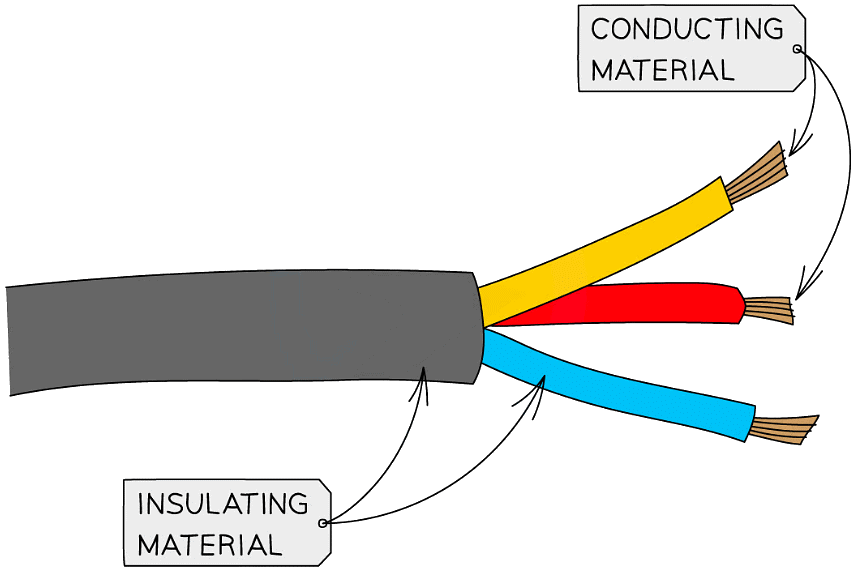
The plastic coating around electrical wires is made from covalent molecules that do not allow a flow of charge
Intermolecular Forces Vs. Covalent Bonds
- Small molecules have covalent bonds joining the atoms together, but intermolecular forces that act between neighbouring molecules
- They have low melting and boiling points as there are only weak intermolecular forces acting between the molecules
- These forces are very weak when compared to the covalent bonds and so most small molecules are either gases or liquids at room temperature
- Often the liquids are volatile
- As the molecules increase in size the intermolecular forces also increase as there are more electrons available
- This causes the melting and boiling points to increase
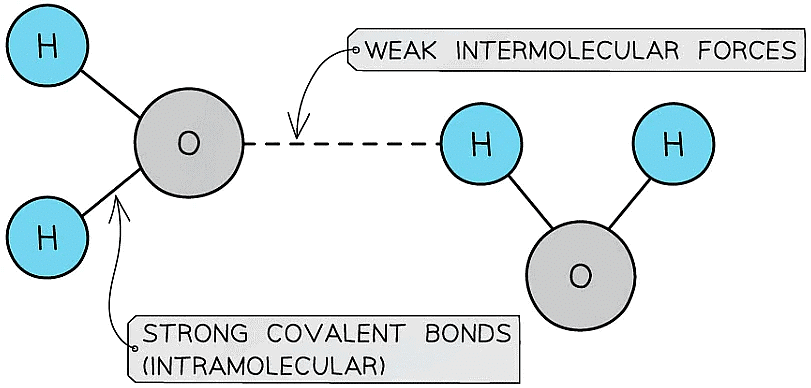
The bonds between hydrogen and oxygen in water are COVALENT, and the attractions between the molecules are INTERMOLECULAR FORCES which are about one tenth as strong as covalent bonds
Polymers
- Polymers are large molecules of high relative molecular mass and are made by linking together large numbers of smaller molecules called monomers
- Each monomer is a repeat unit and is connected to the adjacent units via strong covalent bonds
- The intermolecular forces acting in between polymer chains are larger than those in between simple molecules so polymers are usually solid at room temperature
- Examples of polymers include polythene and polychloroethene, commonly known as PVC
- Many everyday materials such as resins, plastics, polystyrene cups, nylon etc. are polymers
- These are manufactured and are called synthetic polymers
- Nature also produces polymers which are called natural or biological polymers
- Examples include DNA, proteins, silk and wool
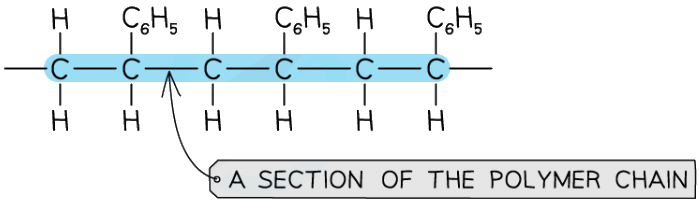
Polymers are made from very long carbon chains with a repeating unit
This diagram shows a short section of polystyrene, a polymer used widely in packaging materials
Drawing Polymers
- Polymers are represented using a specific notation which is shown below using polythene as an example
- You can spell it polythene or polyethene - both are acceptable
- The bonds on either side of the polymer must extend outside the brackets (these are called extension or continuation bonds)
- A small subscript n is written on the bottom right hand side to indicate a large number of repeat units
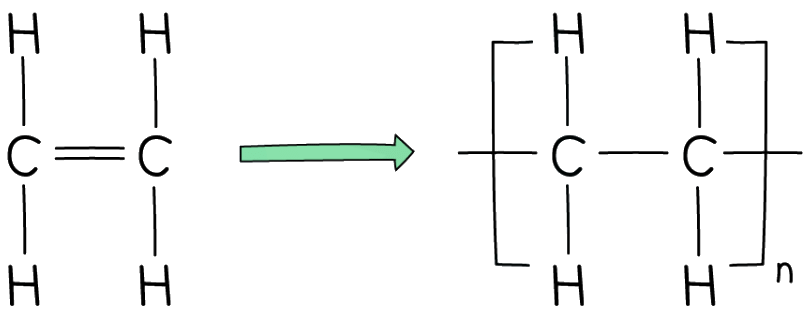
The simplest of all polymers is polyethene
- The relationship between the monomer, repeating unit and polymer is illustrated in this second example using polychloroethene
- Notice that the chlorines do not necessarily have to be drawn 'up' or 'down' from the chain, as long as every carbon has the correct atoms attached
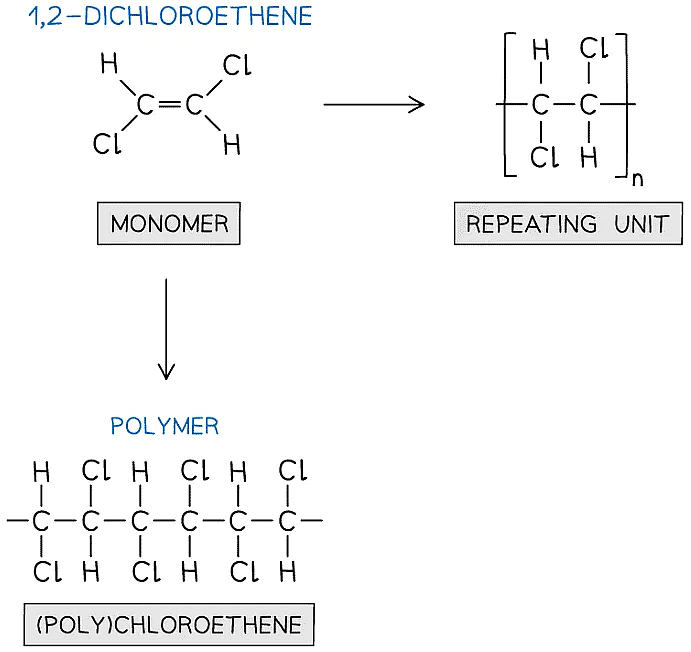
The structure of polychloroethene and its monomer
Giant Covalent Substances
- Covalent bonding can be responsible for substances that have many different structures and therefore different physical properties
- We have already seen how small molecules such as H2O and N2 are simple units made from covalently bonded atoms
- These simple molecules contain fixed numbers of atoms
- Giant covalent structures on the other hand have a huge number of non-metal atoms bonded to other non-metal atoms via strong covalent bonds
- These structures can also be called giant lattices and have a fixed ratio of atoms in the overall structure
- Three common macromolecules you should know about are diamond, graphite and silicon dioxide
Properties: Giant Covalent Substances
- They have high melting and boiling points as they have many strong covalent bonds
- Large amounts of heat energy are needed to overcome these forces and break down bonds
- Most cannot conduct electricity as they do not have free electrons nor charged particles but there are some exceptions such as graphite and graphene
- Diamond, graphite, buckminsterfuller and graphene are all made from carbon
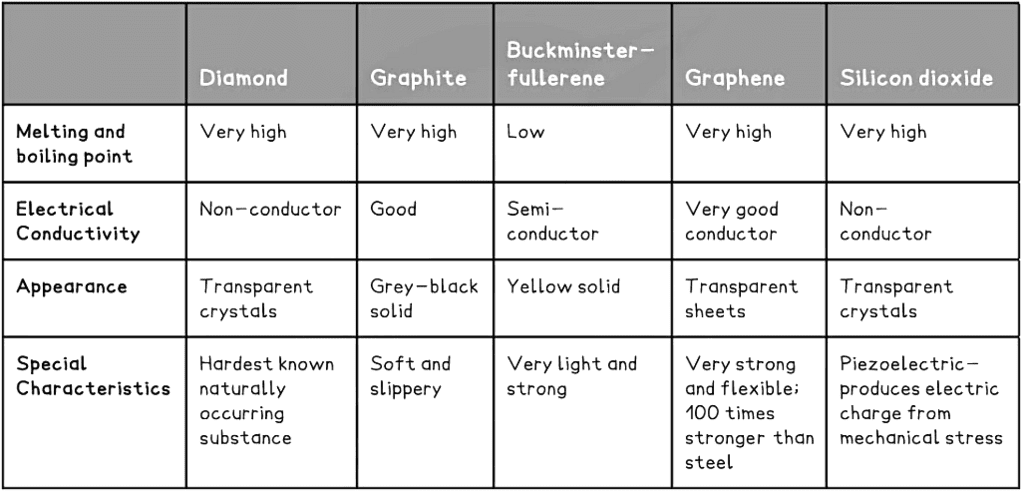
Some giant covalent structures and their properties. Although Buckminsterfullerene is not a giant structure it provides a good illustration of how different forms of the same element, carbon in this case, can form simple molecules and giant lattices
Properties of Metals & Alloys
- Metallic bonds are very strong and are a result of the attraction between the positive metal ions and the negative delocalised electrons within the metal lattice structure
- Metals thus have very high melting and boiling points and are solids at room temperature, with the exception of mercury which is a liquid
- They are usually insoluble in water although some do react with it
- Metals are good conductors of heat and electricity due to the delocalised electrons
- The layers of atoms in metals can slide over each other meaning metals are malleable and can be hammered and bent into shapes or rolled into flat sheets
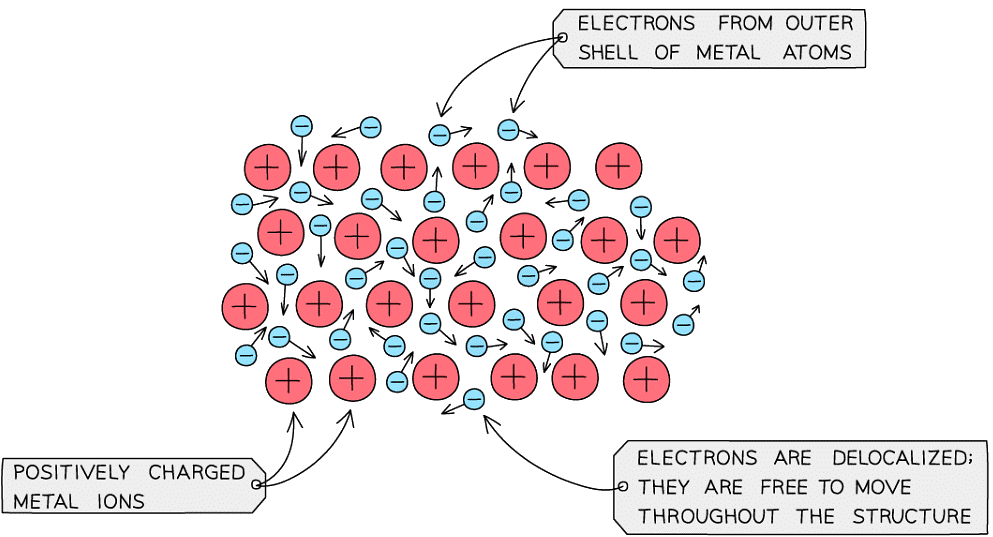
Diagram showing metallic lattice structure with delocalised electrons
Distortion in Alloys
- Alloys are mixtures of metals, where the metals are mixed together physically but are not chemically combined
- They can also be made from metals mixed with non-metals such as carbon
- Alloys often have properties that can be very different to the metals they contain, for example, they can have greater strength, hardness or resistance to corrosion or extreme temperatures
- Alloys contain atoms of different sizes, which distorts the regular arrangements of atoms
- This makes it more difficult for the layers to slide over each other, so they are usually much harder than the pure metal
- Brass is a common example of an alloy which contains 70% copper and 30% zinc
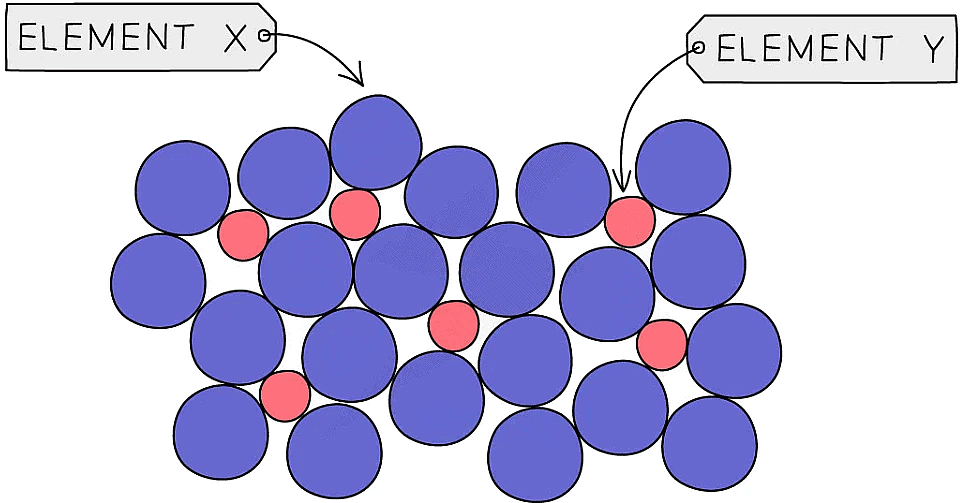
Particle diagram showing a mixture of elements in an alloy. The different sizes of the two types of atoms prevent the layers of atoms from sliding over each other, so the alloy becomes less malleable than the pure metal
Metals as Conductors
Electrical Conductivity
- Metals have free electrons available to move and carry charge throughout the metal lattice structure
- Free electrons can also be called mobile or delocalised
- Electrons entering one end of the metal cause a delocalised electron to displace itself from the other end
- Hence electrons can flow so electricity is conducted
- Copper is used extensively in the production of electrical wiring due to its excellent malleability and electrical conductivity
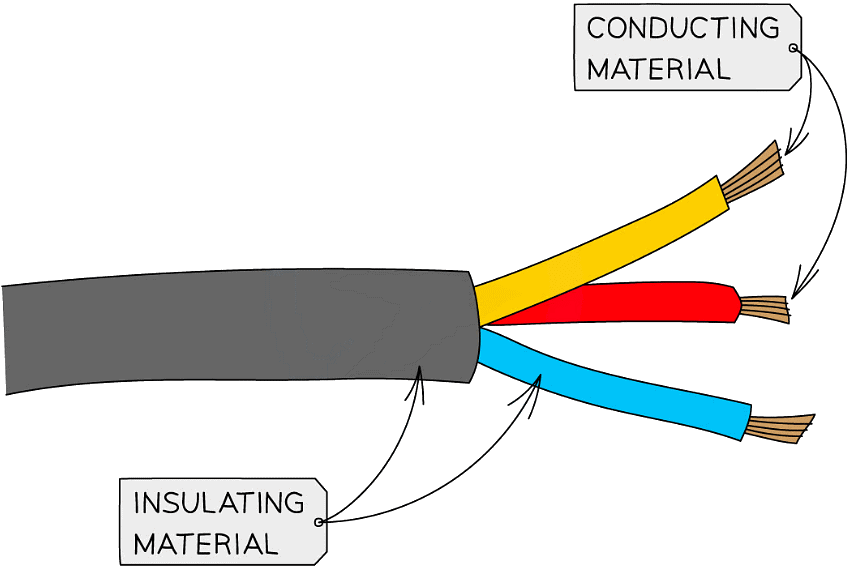
Copper has one of the highest electrical conductivities of any metal
Thermal Conductivity
- Similarly, metals are also good conductors of heat
- The delocalised electrons are free to move and can also carry thermal energy throughout the metal lattice structure
- Some metals are better conductors of heat energy than others
A Table Showing the Different Conductivities of Metals
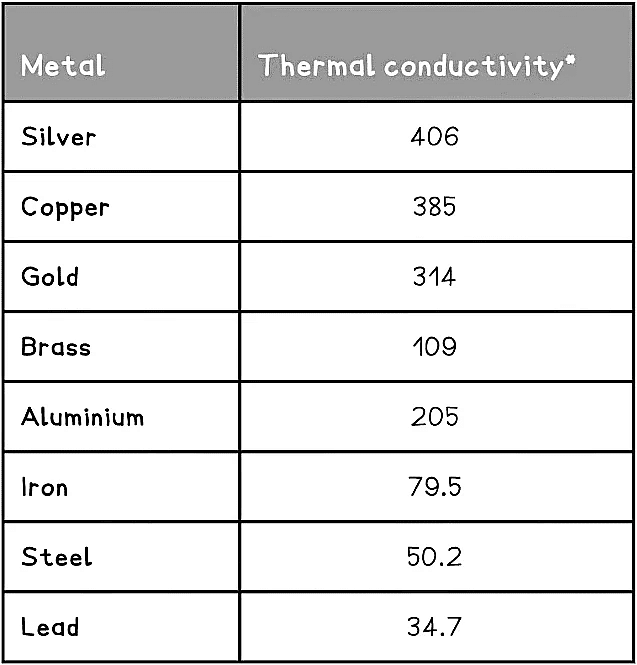
*W/mK = Watts per metre Kelvin is a unit of thermal conductivity
|
75 videos|131 docs|24 tests
|




















