Grade 10 Exam > Grade 10 Notes > Physics for Grade 10 > Atomic Number & Mass Number
Atomic Number & Mass Number | Physics for Grade 10 PDF Download
| Table of contents |

|
| Atomic Number |

|
| Mass Number |

|
| Tip |

|
| Nuclear Notation |

|
Atomic Number
- The number of protons in an atom is called its atomic number (it can also be called the proton number)
- Elements in the periodic table are ordered by their atomic number
- Therefore, the number of protons determines which element an atom is
- The atomic number of a particular element is always the same
- For example:
- Hydrogen has an atomic number of 1. It always has just one proton
- Sodium has an atomic number of 11. It has 11 protons
- Uranium has an atomic number of 92. It has 92 protons
- The atomic number is also equal to the number of electrons in an atom
- This is because atoms have the same number of electrons and protons in order to have no overall charge
Mass Number
- The total number of particles in the nucleus of an atom is called its mass number
- The mass number is the number of protons and neutrons in the atom
- The number of neutrons can be found by subtracting the atomic number from the mass number
Number of Neutron = Mass Number - Atomic Number - For example, if a sodium atom has a mass number of 23 and an atomic number of 11, then the number of neutrons would be 23 – 11 = 12
Tip
You may have noticed that the number of electrons is not part of the mass number. This is because electrons have a tiny mass compared to neutrons and protons. We say their mass is negligible when compared to the particles in the nucleus.
Nuclear Notation
- The mass number and atomic number of an atom are shown by writing them with the atomic symbol
- This is called nuclear notation
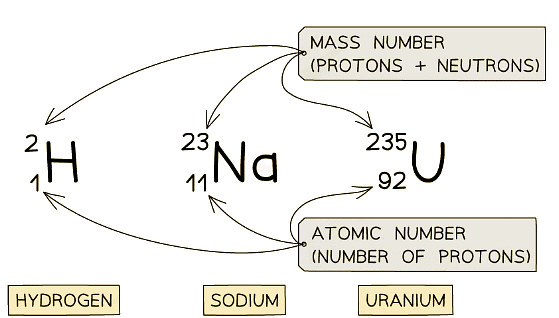
- Here are three examples:
 Examples of nuclear notation for atoms of Hydrogen, Sodium and Uranium
Examples of nuclear notation for atoms of Hydrogen, Sodium and Uranium - The top number is the mass number
- This is equal to the total number of particles (protons and neutrons) in the nucleus
- The lower number is the atomic number
- This is equal to the total number of protons in the nucleus
- The atomic and mass number of each type of atom in the examples above is shown in this table:

Question for Atomic Number & Mass NumberTry yourself:The element symbol for gold is Au. How many protons, neutrons and electrons are in the gold atom?
 View Solution
View Solution
The document Atomic Number & Mass Number | Physics for Grade 10 is a part of the Grade 10 Course Physics for Grade 10.
All you need of Grade 10 at this link: Grade 10
|
124 videos|149 docs|37 tests
|

|
Explore Courses for Grade 10 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
Related Searches