Grade 10 Exam > Grade 10 Notes > Physics for Grade 10 > The Plum Pudding Model
The Plum Pudding Model | Physics for Grade 10 PDF Download
| Table of contents |

|
| Introduction |

|
| Early Models of the Atom |

|
| JJ Thompson’s Plum Pudding Model |

|
| Tip |

|
Introduction
- Scientists understanding of what atoms are has changed through time
- Different models have been developed, and then replaced as new evidence from experiments is discovered
- A model is a way of describing something in order to explain the way it behaves
Early Models of the Atom
- Greek and Indian philosophers were the first to try and describe the idea of everything being made up of smaller parts
- The Greek philosopher, Democritus, thought that although objects could be cut into smaller pieces, the smallest possible piece would be indivisible (it could not be cut any further)
- The Greek word for ‘indivisible’ is atomos
 Democritus thought about cutting objects into smaller and smaller pieces until they were indivisible
Democritus thought about cutting objects into smaller and smaller pieces until they were indivisible - Therefore, atoms were initially thought to be tiny spheres that could not be divided before the discovery of the electron
- Later models described the atom as small solid spheres
- The Greek word for ‘indivisible’ is atomos
JJ Thompson’s Plum Pudding Model
- At the end of the 19th Century, Physicist Joseph Jon Thompson discovered the existence of electrons
- This new evidence meant a better model of the atom was required
- Thompson proposed the Plum Pudding model
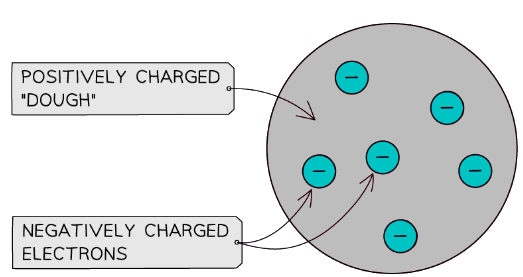
- The atom was thought to consist of negatively charged electrons (the ‘plums’) in a positively charged ‘dough’ or ‘pudding’
 J J Thomson thought of the atom as being a positively charged mass embedded with small negatively charged electrons – a bit like a plum pudding
J J Thomson thought of the atom as being a positively charged mass embedded with small negatively charged electrons – a bit like a plum pudding - It was known that electrons were much smaller than atoms, so it made sense that they should be embedded within the larger atom
- Since electrons have a negative charge, it was reasoned that the rest of the atom would be positive, making the atom neutral overall
- The atom was thought to consist of negatively charged electrons (the ‘plums’) in a positively charged ‘dough’ or ‘pudding’
Tip
For the exam you need to be able to describe the features of JJ Thompson’s Plum Pudding model, but you do not need to know how electrons were discovered (this is covered at A Level), or about very early atomic models.
The document The Plum Pudding Model | Physics for Grade 10 is a part of the Grade 10 Course Physics for Grade 10.
All you need of Grade 10 at this link: Grade 10
|
124 videos|149 docs|37 tests
|

|
Explore Courses for Grade 10 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
Related Searches