Grade 10 Exam > Grade 10 Notes > Chemistry for Grade 10 > Electrolysis of Molten Ionic Compounds
Electrolysis of Molten Ionic Compounds | Chemistry for Grade 10 PDF Download
Electrolysis of Simple Ionic Compounds
- Lead(II) bromide is a binary ionic compound meaning that it is a compound consisting of just two elements joined together by ionic bonding
- When these compounds are heated beyond their melting point, they become molten and can conduct electricity as their ions can move freely and carry the charge
- These compounds undergo electrolysis and always produce their corresponding element
- To predict the products of any binary molten compound first identify the ions present
- The positive ion will migrate towards the cathode and the negative ion will migrate towards the anode
- Therefore the cathode product will always be the metal and the product formed at the anode will always be the non-metal
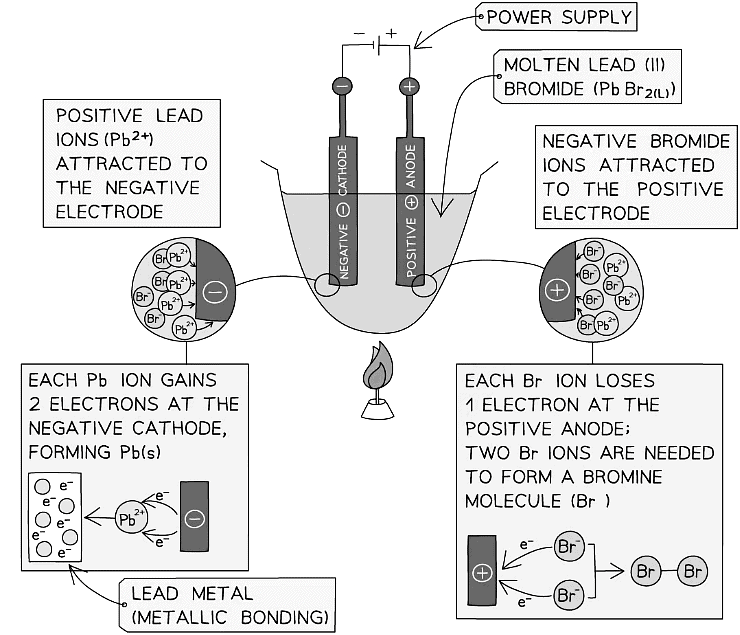 Diagram showing the electrolysis of lead (II) bromide
Diagram showing the electrolysis of lead (II) bromide
Method
- Add lead(II) bromide into a crucible and heat so it will turn molten, allowing ions to be free to move and conduct an electric charge
- Add two graphite rods as the electrodes and connect this to a power pack or battery
- Turn on the power pack or battery and allow electrolysis to take place
- Negative bromide ions move to the positive electrode (anode) and lose two electrons to form bromine molecules. There is bubbling at the anode as brown bromine gas is given off
- Positive lead ions move to the negative electrode (cathode) and gain electrons to form grey lead metal which deposits on the bottom of the electrode
Electrode Products:
Anode: Bromine gas
Cathode: Lead metal
The document Electrolysis of Molten Ionic Compounds | Chemistry for Grade 10 is a part of the Grade 10 Course Chemistry for Grade 10.
All you need of Grade 10 at this link: Grade 10
|
73 videos|87 docs|21 tests
|
Related Searches















