Osmosis | Biology for Grade 10 PDF Download
| Table of contents |

|
| Introduction |

|
| Osmosis Across Living Cells |

|
| Plant Cells |

|
| Animal Cells |

|
| Required Practical - the Effect of Osmosis on Plant Tissue |

|
Introduction
Osmosis is the diffusion of water molecules, from a region where the water molecules are in higher concentration, to a region where they are in lower concentration, through a partially permeable membrane.
A dilute solution contains a high concentration of water molecules, while a concentrated solution contains a low concentration of water molecules.
Osmosis refers to the movement of water molecules only.
The slideshow shows an example of osmosis showing the direction of movement of water between two different concentrations of sugar solutions.
Step one
The beaker contains water and sugar molecules

Step two
Water molecules pass through from solution one to two
When the concentration of water is the same on both sides of the membrane, the movement of water molecules will be the same in both directions. There will be no net movement of water molecules. In theory, the level of solution two will rise, but this will be opposed by gravity and will be dependent on the width of the container.
Similar observations will be made with solutions containing different solutes, for instance, salt instead of sugar.
Osmosis Across Living Cells
- Cells contain dilute solutions of ions, sugars and amino acids.
- The cell membrane is partially permeable.
- Water will move into and out of cells by osmosis.
Plant Cells
- Isolated plant cells placed in a dilute solution or water will take in water by osmosis. Root hair cells, if the soil is wet or moist, will also take up water by osmosis. Leaf cells of land plants, unless it is raining or the humidity is high, will have a tendency to lose water.
- Plant cells have a strong cellulose cell wall outside the cell membrane. The cell wall is fully permeable to all molecules and supports the cell and stops it bursting when it gains water by osmosis.
If plant cells are placed in solutions of increasing solute concentration:
Pure water
In pure water, the cell contents (the cytoplasm and vacuole) push against the cell wall and the cell becomes turgid.
Fully turgid cells support the stems of non-woody plants.

Concentrated solution
In a more concentrated solution, the cell contents lose water by osmosis. They shrink and pull away from the cell wall. The cell becomes flaccid. It is becoming plasmolysed.
Highly-concentrated solution
- In a very concentrated solution, the cell undergoes full plasmolysis as the cells lose more water.
- Plants would be exposed to higher concentrations of solutes if there was less water in the soil - for instance, if plants were not watered, or plants in drought conditions. Plant cells would then lose water by osmosis.
- Aquatic, freshwater plants placed in the sea, or a seaweed in a rock pool where the water evaporated in the Sun, would also lose water by osmosis.
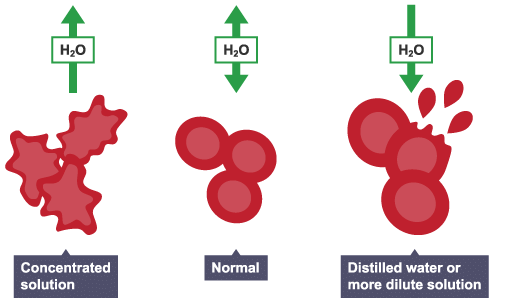
Animal Cells
Animal cells also take in and lose water by osmosis. They do not have a cell wall, so will change size and shape when put into solutions that are at a different concentration to the cell contents.
For example, red blood cells could:
- lose water and shrink
- gain water, swell and burst in a more dilute solution

In animals, the concentration of body fluids – blood plasma and tissue fluid – must be kept within strict limits – if cells lose or gain too much water by osmosis, they do not function efficiently.
Required Practical - the Effect of Osmosis on Plant Tissue
Investigate the effect of a range of concentrations of salt or sugar solutions on the mass of plant tissue
Scientists investigate the effects of osmosis on living cells. They either:
- Observe (with a microscope),cells or tissues placed in solutions of different concentration.
- Measure changes in cylinders or discs of fresh potato or beetroot. Cylinders will have a larger mass than discs, so scientists will have larger measurements to work with.
The following experiment investigates the effect of different concentrations of sucrose on potato tissue. It could also be carried out using salt – sodium chloride solution – instead of sucrose.
Aims of the experiment
- To investigate the effect of a range of sucrose solutions on the mass of potato cylinders.
- To determine the concentration of the cell sap of potato cells.
Method
- Prepare a range of sucrose (sugar) solutions. The concentration of a solution is measured in moles per cubic decimetre written as mol dm-3. For example, in this experiment your range could be from 0.2 mol dm-3 to 1.0 mol dm-3. A 1.0 mol dm-3 solution of sucrose will contain up to 342 g of sucrose per dm-3. A 1.0 mol dm-3 solution of a substance contains one mole of the substance per dm3 of a solution, or one mole per litre of solution.
- Set up a series of boiling tubes with each of these solutions. Also, set up one containing distilled water. This will have a concentration of sucrose of 0.0 mol dm-3 and will act as the control in the experiment.
- Make sure each tube is labelled with the concentration.
- Carry out the investigation. Prepare a blank results table before you begin. Make sure when weighing the potato cylinders, that their masses are not mixed up when recording them. Each cylinder will have a different mass before and after the investigation.
- For each sucrose concentration, repeat the investigation for several potato cylinders. This allows you to make the experiment more repeatable – not all potato cylinders might behave in the same way. Making a series of repeat experiments means that any anomalous results can be identified and ignored when a mean is calculated.
Risks
- Make sure that the potato is placed on a ceramic tile when using the cork borer – do not cut the potato cylinders towards your hand.
- Care must be taken when using the scalpel.
- Wear eye protection when using chemical solutions.
This experiment shows the effect of osmosis on plant tissue. The cylinders will decrease or increase in mass if they lose or gain water by osmosis.
The effects on plant tissues at a cellular level can be observed using a microscope. Another way of looking at osmosis in plant cells is to mount a piece of onion skin, or beetroot on microscope slides in drops of different concentrations of sugar or salt. Observe the cells for a few minutes. It is easy to see the process of plasmolysis in beetroot because the cell sap is red.
|
110 videos|93 docs|9 tests
|

|
Explore Courses for Grade 10 exam
|

|