Enzymes Structure, Classification and Function | Science for ACT PDF Download
| Table of contents |

|
| Enzymes |

|
| Enzyme Structure |

|
| Enzymes Classification |

|
| Enzyme Cofactors |

|
| Enzyme Reaction Mechanism |

|
| Enzyme-Substrate Interactions |

|
| Enzyme Action |

|
| Concentration and Type of Substrate |

|
Enzymes
"Enzymes can be defined as biological macromolecules that facilitate and accelerate biochemical reactions."
Enzymes are primarily protein-based catalysts essential for a variety of processes. They play a crucial role in metabolic and other cellular reactions necessary for sustaining life.
Enzymes initiate metabolic processes by interacting with specific molecules known as substrates. Through enzymatic activity, substrates are transformed into distinct molecules called products.
Enzyme regulation is significant in clinical diagnosis due to their involvement in maintaining vital life processes. While most enzymes consist of protein components, there is a class of RNA catalysts called ribozymes. Ribozymes, derived from ribonucleic acid enzymes, include many RNA molecules that catalyze reactions involving their own bonds or other RNAs.
Enzymes are present in all tissues and bodily fluids. Intracellular enzymes catalyze reactions within metabolic pathways, while enzymes on the plasma membrane respond to cellular signals. Enzymes in the circulatory system regulate blood clotting. The functions of enzymes are fundamental to numerous essential life processes.
Enzyme Structure
Enzymes possess a linear arrangement of amino acids that ultimately determine their three-dimensional structure. The specific sequence of amino acids dictates the overall structure of the enzyme, which, in turn, defines its catalytic activity. When exposed to high temperatures, the enzyme's structure undergoes denaturation, resulting in the loss of its catalytic function, often associated with temperature sensitivity.
Enzymes typically exhibit larger sizes compared to their substrates, ranging from 62 amino acid residues to an average of 2500 residues, as seen in fatty acid synthase. The catalytic activity of the enzyme primarily occurs in a specific region of the structure, adjacent to the binding sites. These binding and catalytic regions collectively form the active site of the enzyme. While most enzymes are protein-based, a limited number of ribozymes function as RNA-based biological catalysts, often interacting with proteins in complex formations.
Enzymes Classification
- Oxidoreductases
- Transferases
- Hydrolases
- Lyases
- Isomerases
- Ligases
In the past, enzymes were typically named after their discoverers. However, as research progressed, a more comprehensive classification system was established.
According to the International Union of Biochemists (IUB), enzymes are now categorized into six functional classes based on the specific type of reaction they catalyze. These six classes of enzymes include hydrolases, oxidoreductases, lyases, transferases, ligases, and isomerases.
Listed below is the classification of enzymes discussed in detail:
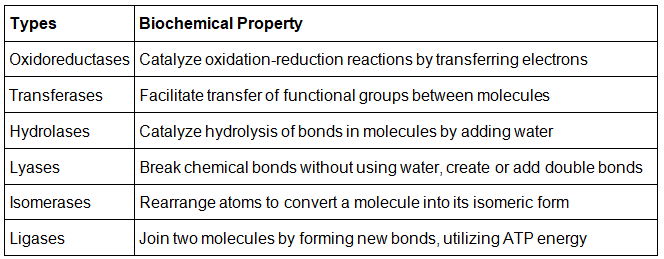
Oxidoreductases
- Oxidoreductases are enzymes that facilitate oxidation and reduction reactions. An example is pyruvate dehydrogenase, which catalyzes the oxidation of pyruvate to acetyl coenzyme A.
Transferases
- Transferases are enzymes that catalyze the transfer of a chemical group from one compound to another. An example is transaminase, which transfers an amino group from one molecule to another.
Hydrolases
- Hydrolases are enzymes that catalyze the hydrolysis of a bond. For instance, pepsin is an enzyme that hydrolyzes peptide bonds in proteins.
Lyases
- Lyases are enzymes that catalyze the breaking of bonds without the use of hydrolysis or transfer of functional groups. Aldolase, an enzyme in glycolysis, catalyzes the splitting of fructose-1,6-bisphosphate into glyceraldehyde-3-phosphate and dihydroxyacetone phosphate.
Isomerases
- Isomerases are enzymes that catalyze the conversion of a compound into its isomeric form. An example is phosphoglucomutase, which catalyzes the conversion of glucose-1-phosphate to glucose-6-phosphate during glycogenolysis.
Ligases
- Ligases are enzymes that catalyze the joining or association of two molecules, typically using energy from ATP. DNA ligase, for example, facilitates the joining of DNA fragments by forming phosphodiester bonds.
Enzyme Cofactors
Cofactors are non-protein substances that associate with enzymes and are essential for their functioning. The protein component of an enzyme without a cofactor is referred to as the apoenzyme. When the cofactor is present, the enzyme and cofactor together form the holoenzyme.
There are three types of cofactors found in enzymes:
- Prosthetic groups: These cofactors are tightly bound to the enzyme at all times. An example is FAD (flavin adenine dinucleotide), which serves as a prosthetic group in numerous enzymes.
- Coenzymes: Coenzymes are cofactors that bind to the enzyme only during the catalytic process and are detached at other times. NAD (nicotinamide adenine dinucleotide) is a common example of a coenzyme.
- Metal ions: Certain enzymes require metal ions at their active sites to facilitate catalysis through the formation of coordinate bonds. Zinc, for instance, serves as a metal ion cofactor for a variety of enzymes.
Examples of Enzyme Applications
Beverages
- The production of alcoholic beverages through fermentation is influenced by various factors, including the type of plant material used and the specific enzymes employed. Different fermented products, such as beer, wines, and other drinks, can be created using materials like grapes, honey, hops, wheat, cassava roots, and potatoes.
Food Products
- Fermentation plays a significant role in everyday life, and bread is an excellent example of this process. When making bread, a small amount of yeast and sugar are mixed with the dough. The enzymes in the yeast then catalyze the fermentation of sugar, resulting in the release of carbon dioxide gas. This gas is responsible for the leavening effect, giving bread its desired texture.
Drug Action
- Drugs can interact with enzymes and either inhibit or promote their activity by targeting the active sites. This modulation of enzyme action is a common mechanism employed by drugs to achieve their desired effects.
Enzyme Reaction Mechanism
In order for a reaction to occur, two molecules must collide with the correct orientation and sufficient energy. This energy required to overcome the reaction barrier is known as activation energy.
Enzymes have an active site, which is a specific region on the enzyme molecule with a defined shape and functional groups that can bind to the reactant molecules. The reactant molecule that binds to the enzyme is called the substrate. When the substrate and enzyme come together, they form an intermediate complex with a lower activation energy, facilitating the reaction without the need for additional catalysts.
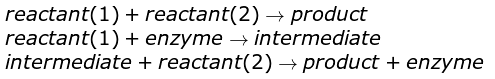

Enzymes function by catalyzing chemical reactions, which initiate with the binding of the substrate to the active site of the enzyme. The active site is a precise region on the enzyme that interacts and binds specifically with the substrate.
Enzyme-Substrate Interactions
Enzymes, being high molecular weight proteinous compounds, act as biocatalysts in various life processes by enhancing chemical reactions. The active site of an enzyme, which contains specific functional groups such as -SH and -COOH, provides a surface for the substrate to bind. The substrate, with an opposite charge to the enzyme, fits into these spaces like a key fitting into a lock, forming the enzyme-substrate complex.
Induced-Fit Model
The induced-fit model describes the favorable interaction between the substrate and enzyme. Weak initial interactions induce rapid conformational changes, strengthening the binding and bringing the catalytic sites close to the substrate bonds.
Major Mechanisms of Catalysis
- Catalysis by Bond Strain: Structural rearrangements induce strain in substrate bonds, facilitating the attainment of the transition state.
- Covalent Catalysis: Substrate orientation leads to the formation of a covalent intermediate between the enzyme and substrate, as observed in proteolysis by serine proteases.
- Catalysis Involving Acids and Bases: Acidic or basic groups, such as glutamate, participate in catalytic events.
- Catalysis by Orientation and Proximity: Enzyme-substrate interactions bring reactive groups in close proximity to each other, promoting catalysis.
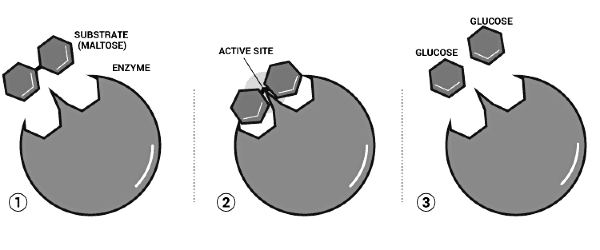
Enzyme Action and Nature
Upon binding of the substrate (S) to the active site, an intermediate enzyme-substrate complex (ES) is formed, leading to the production of the product (P) and the release of the enzyme (E). The enzyme specifically accommodates a substrate with a particular structure. By providing a surface for the substrate, enzymes lower the activation energy of the reaction. The transition state represents the intermediate state where the substrate binds to the enzyme. Through bond breaking and formation, the substrate binds to the enzyme (which remains unchanged), resulting in the conversion to the product. The enzyme is then freed and can bind to other substrates, continuing the catalytic cycle until the reaction is complete.
The enzyme action basically happens in two steps:
Step1: Combining of enzyme and the reactant/substrate.
E+S → [ES]
Step 2: Disintegration of the complex molecule to give the product.
[ES]→E+P
Thus, the whole catalyst action of enzymes is summarized as:
E + S → [ES] → [EP] → E + P
Enzyme Action
Biological Catalysts and Enzymes
Catalysis in Chemical Reactions
- Catalysts are substances that facilitate chemical reactions without being consumed themselves. They alter/enhance the rate of a chemical reaction without undergoing any change in quantity or chemical properties. One such catalyst found in living organisms is enzymes, commonly known as biological catalysts. Enzymes play a vital role in accelerating reactions within the body.
Specificity and Active Site
- Enzymes, as biological catalysts, exhibit high specificity, catalyzing a single chemical reaction or closely related reactions. The precise structure and active site of an enzyme determine its specificity. Substrate molecules bind to the active site of an enzyme through noncovalent interactions, including ionic, hydrogen bonds, and hydrophobic interactions. Enzymes lower the activation energy required for reactions, allowing them to progress towards equilibrium at a faster rate compared to uncatalyzed reactions.
Regulation and Cellular Response
- Both eukaryotic and prokaryotic cells employ allosteric regulation to respond to internal cellular changes. This regulatory mechanism enables enzymes to respond and adapt to fluctuations within the cell, maintaining proper cellular function and metabolic balance.
Nature of enzyme action and factors affecting the enzyme activity are discussed below
Factors Affecting Enzyme Activity
Enzyme activity is influenced by various factors that determine the conditions required for optimal performance. The key factors include temperature, pH, and substrate concentration.
- Temperature and Denaturation
- The effect of temperature on enzyme activity is significant. Typically, as temperatures rise, enzyme activity increases. Enzymes function best at moderate temperatures, which are suitable for cellular environments. However, beyond a certain point, high temperatures lead to denaturation and a sharp decline in enzyme activity. Enzymes in diluted solutions, especially purified enzymes, are more susceptible to denaturation compared to enzymes in crude extracts. Prolonged incubation times can also result in enzyme denaturation, making it important to use shorter incubation periods when measuring initial velocities of enzyme reactions. The International Union of Biochemistry recommends a standard assay temperature of 30 °C for enzyme studies.
- pH and Ionic State
- Enzymes are highly sensitive to changes in pH. Most enzymes exhibit optimal activity near neutral pH values, typically ranging between pH 5 and 7. Deviations from the optimal pH can cause alterations in the ionic state of amino acid residues within the enzyme, affecting catalysis and substrate binding. Some enzymes can operate at pH levels outside this range, with a few functioning at pH values above 9 or below 5.
- Substrate Concentration
- The concentration of the substrate also plays a crucial role in enzyme activity. At lower substrate concentrations, the rate of the reaction is dependent on the substrate concentration. However, once the substrate concentration reaches a certain level, further increases do not significantly affect the reaction rate.
- Active Site and Catalysis
- Enzymatic catalysis relies on the activity of amino acid side chains within the enzyme's active site. The active site is often a specific region, such as a cleft or pocket, formed by amino acids involved in catalysis and substrate binding. These amino acids are not contiguous along the primary amino acid sequence but rather assemble in the correct conformation through three-dimensional folding. Polar amino acids like aspartate, cysteine, glutamate, histidine, serine, and lysine are commonly found in the active sites of enzymes. Only a few essential amino acid residues directly participate in the bond formation leading to product formation. Additionally, certain amino acid residues, such as glutamate, aspartate, and histidine, can act as proton acceptors or donors.
- Optimum Temperature and pH
- Enzymes require specific temperature and pH conditions for optimal activity. The temperature or pH at which an enzyme exhibits maximum activity is known as the optimum temperature or optimum pH, respectively. Enzymes, being protein compounds, can undergo molecular structure alterations at temperatures or pH levels above or below their optimum. Generally, enzymes have an optimum pH in the range of 5 to 7.
 (i) Optimum T°
(i) Optimum T°
(ii) The greatest number of molecular collisions
(iii) human enzymes = 35°- 40°C
(iv) body temp = 37°C
(v) Heat: increase beyond optimum T°
(vi) The increased energy level of molecule disrupts bonds in enzyme & between enzyme & substrate H, ionic = weak bonds
(vi) Denaturation = lose 3D shape (3° structure)
(vii) Cold: decrease T°
(viii) Molecules move slower decrease collisions between enzyme & substrate
- Enzymes require specific temperature and pH conditions for optimal activity. The temperature or pH at which an enzyme exhibits maximum activity is known as the optimum temperature or optimum pH, respectively. Enzymes, being protein compounds, can undergo molecular structure alterations at temperatures or pH levels above or below their optimum. Generally, enzymes have an optimum pH in the range of 5 to 7.
Concentration and Type of Substrate
Enzyme Saturation and Velocity
Enzymes have a saturation point where their activity ceases once all the enzyme molecules are occupied by substrate molecules. Initially, as substrate is added, the velocity of enzyme action increases. However, at the saturation point, where substrate molecules exceed the available free enzyme, the velocity remains constant.
Effect of Substrate Type: Inhibitors
The type of substrate can influence enzyme activity. Certain chemicals can inhibit enzyme activity by binding to the enzyme's active site. These substances are called inhibitors. Competitive inhibitors structurally resemble the specific substrate and compete for the active site, thereby inhibiting enzymatic activity. This concept is utilized in the treatment of bacterial infectious diseases.
Salt Concentration and Enzyme Function
Impact of Salinity Changes
- Changes in salt concentration, which involve the addition or removal of cations (+) and anions (–), can affect enzyme function. Such changes disrupt bonds, including those between charged amino acids, leading to the disruption of the enzyme's three-dimensional shape. This alteration in the secondary and tertiary structure can result in protein denaturation. Enzymes are generally intolerant of extreme salinity conditions.
Functions of Enzymes
Signal Transduction
- Enzymes play a role in signal transduction processes. One example is protein kinase, an enzyme that catalyzes the phosphorylation of proteins, facilitating signaling within cells.
Breakdown of Large Molecules
- Enzymes assist in breaking down large molecules into smaller substances that can be easily absorbed by the body.
Energy Generation
- Enzymes are involved in generating energy within the body. ATP synthase, an enzyme, participates in the synthesis of ATP, the energy currency of cells.
Ion Movement Across Membranes
- Enzymes contribute to the movement of ions across plasma membranes, facilitating important cellular processes.
Biochemical Reactions
- Enzymes catalyze a variety of biochemical reactions, including oxidation, reduction, and hydrolysis, enabling the elimination of non-nutritive substances from the body.
Regulation of Cellular Activities
- Enzymes aid in the reorganization of the internal structure of cells, playing a crucial role in regulating cellular activities.
|
486 videos|517 docs|337 tests
|

|
Explore Courses for ACT exam
|

|
 (i) Optimum T°
(i) Optimum T°















